Radiopharmaceuticals: Prostate cancer diagnostic revenues currently in focus, but the opportunity in therapeutics could be larger
Australian Investors have witnessed the rise of Telix Pharmaceuticals ASX:TLX, which listed in 2017 with a market capitalisation of AU$128m and is now valued at AU$3.2 billion. The company's PSMA-PET[i] diagnostic, Illucix, has garnered significant investor attention, with expectations of reaching approximately US$800 million in peak sales, representing a significant portion of the ~US$2 billion total addressable market (TAM) for PSMA-PET diagnostics. However, the prevailing market focus on TLX's PSMA-PET diagnostic revenue has overshadowed the significant clinical and revenue potential of radiopharmaceuticals to not just diagnose, but also treat cancer.
In this wire we will go beyond the immediate opportunity in diagnostics and review the landscape for prostate cancer radiotherapeutics.
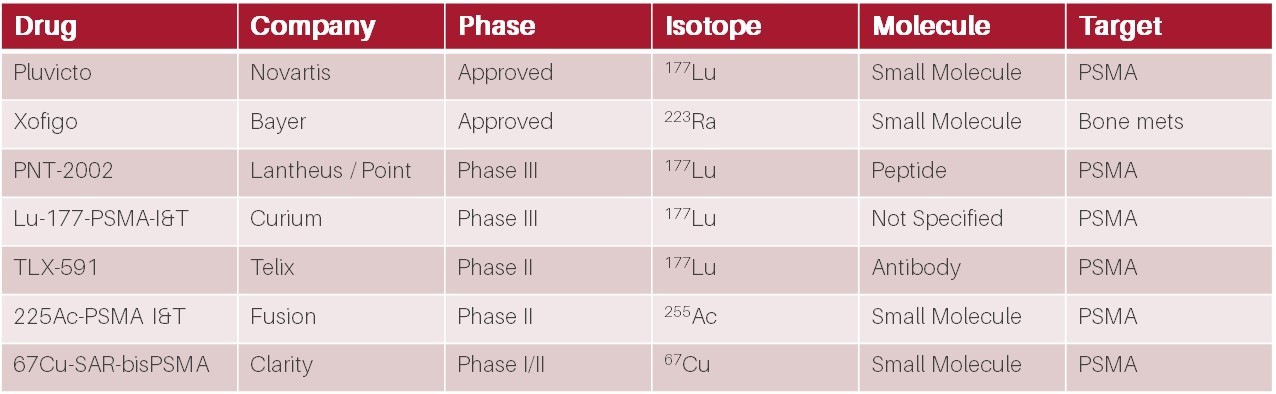
A selection of later-stage prostate cancer radiopharmaceutical therapies under development. Source: Biomedtracker / HB Biotech analysis
The Rise of Prostate Cancer Theranostics
Telix is one of a growing cohort of biotech companies developing “Theranostic” products. Theranostic is a term used in nuclear medicine to describe using the same cancer-targeting technology to first image a cancer using a lower-energy diagnostic isotope such as 68Gallium then, using the same cancer-targeting technology, switching to a higher-energy, ionising radioisotope such as 177Lutetium capable of killing cancer cells. This approach is particularly well suited to metastatic disease that has spread throughout the body and cannot be effectively targeted using traditional external-beam radiation therapy.
Currently there is only one PSMA-targeting radiotherapeutic approved by the FDA: Novartis’ NYSE:NVS Pluvicto. Novartis gained the rights to Pluvicto when it acquired Endocyte NASDAQ:ECYT in 2018 for US$2.1b. At this stage, Pluvicto was in Phase 3 clinical trials and had yet to be approved by the FDA.
Since Pluvicto’s approval in 2022, Novartis has struggled to keep up with demand due to supply constraints. Despite this, analysts forecast sales of Pluvicto to be >US$2b p.a. in its initial indication of PSMA-positive mCRPC[ii] patients who have tried an androgen receptor inhibitor and at least one taxane-based chemotherapy regimen. However, the revenue potential in prostate cancer could be substantially greater given initial results from Novartis’ PSMAfore trial (in pre-chemo patients) suggesting that Pluvicto’s eligible patient pool could expand from ~27,000 to 42,000 patients in the US alone. At ~US$250,000 for a course of Pluvicto treatment, this represents a TAM of >US$10b.
However Pluvicto is not the only PSMA-targeted radiotherapy in development. Close behind, Point Biopharma NASDAQ:PNT is developing PNT-2002, a 177Lu-PSMA radiotherapy product substantially similar to Pluvicto, that is in late-stage Phase 3 clinical trials. While PNT-2002 is essentially a “me-too” product with little differentiation and second to market, analysts currently expect it to generate ~US$1b p.a. in peak sales. This was enticing enough for Lantheus (NASDAQ:LNTH / TLX’s main competitor in the PSMA-PET diagnostics market) to acquire the rights to PNT-2002 earlier this year for US$260m upfront, up to US$1.8m in milestone payments, as well as a 20% royalty on net sales, further highlighting the opportunity in prostate cancer radiotherapeutics.
Clinical Benefit of Radiotherapeutics
The reason for excitement is the significant survival benefit that these therapies have demonstrated. In its Phase 3 VISION trial, Pluvicto demonstrated an overall survival benefit of 15.3 months compared to 11.3 months for patients receiving standard of care. While making cross-trial comparisons is always fraught with danger, initial results indicate that the survival benefit of Telix’s TLX-591 may be somewhere between 28 months (post-chemo) to 48 months (pre-chemo, shown below) – a significant increase on 15.3 months.
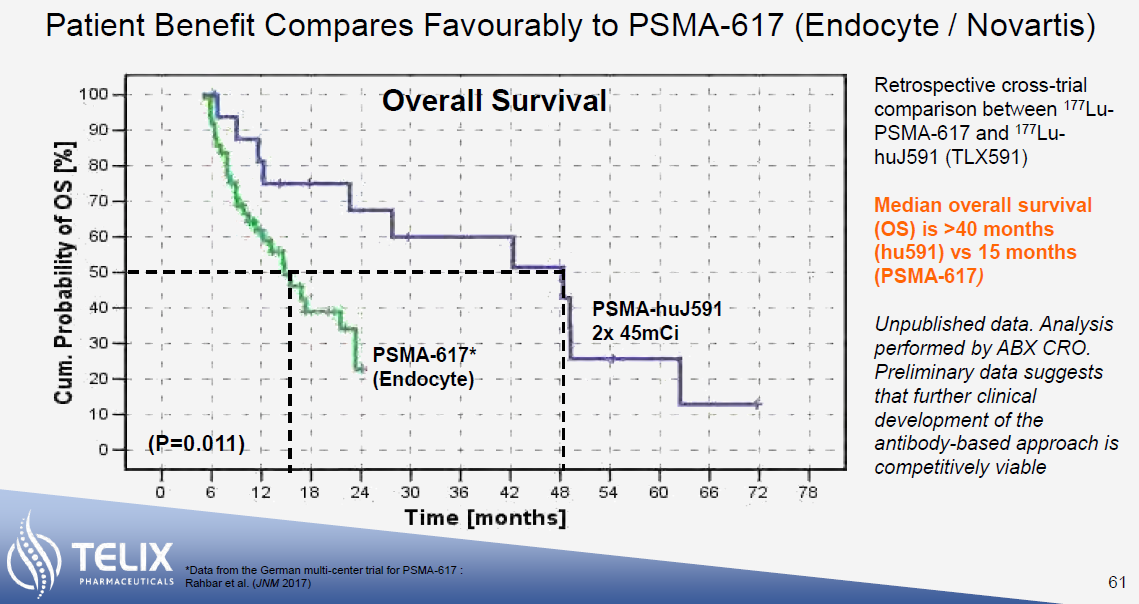
Whereas both Pluvicto and TLX-591 both use 177Lutetium as the therapeutic radioisotope, TLX uses an antibody for PSMA targeting compared to Pluvicto’s small molecule. While the antibody approach potentially leads to greater efficacy, this comes at the expense of greater haematological toxicity. TLX is expected to read out initial results from its ProstACT SELECT trial of TLX-591 in mCRPC patients by the end of 2023. This should provide further insight into the safety and tolerability of TLX-591, the results of which will likely form part of an IND[iii] in the United States.
Further approaches to prostate cancer radiotherapeutics
Also in the Australian market, Clarity Pharmaceuticals ASX:CU6 is developing a prostate cancer therapeutic utilising 67Cu as the therapeutic radioisotope. While 67Cu has traditionally proven difficult to use, resulting in limited investment in the infrastructure for medical grade commercial supply to date, the emergence of Clarity’s SAR-technology has prompted the first commercial supply of 67Cu by Northstar Medical Radioisotopes. Clarity utilises a bis-PSMA targeting moiety (two PSMA binding domains) and, while still very early days, has recently shown remarkable PSA reductions from a single dose in its Phase 1/2 SECuRE trial being conducted at sites in the United States.
In addition to 67Cu-SAR-bisPSMA, Clarity is also developing 67Cu-SAR-Bombesin which is potentially capable of detecting and treating PSMA negative prostate cancer – a patient population that is ineligible for PSMA-targeted therapies such as Pluvicto and TLX-591. Estimates suggest that ~20% of prostate cancer patients either do not express PSMA, or express PSMA at very low levels. While this market may be smaller than for PSMA-targeted therapies, currently there are few therapeutic options for these patients and Clarity is the only company developing a radiotherapeutic option, representing a compelling market opportunity.
Also of interest is Fusion Pharmaceutical’s NASDAQ:FUSN 225Ac-PSMA which uses an alpha-particle emitting isotope (225Ac), as opposed to the beta-emitters used by the companies discussed above. The attraction of using an alpha-emitter is the short range, but much higher energy compared to beta-emitters. In simple terms, greater cancer killing power but with a very short blast radius. Current thinking is that radiotherapy with alpha-emitters will likely be complementary to beta-emitters. The results of FUSN’s Phase 2 TATIST trial are expected in Q1 2024.
Just scratching the surface
While we have only briefly discussed a selection of prostate cancer radiotherapies, we acknowledge there is a burgeoning pipeline of radiopharmaceuticals targeting a range of different cancers – but to review all these would take much more than one wire!
So, while the clinical utility and revenues of diagnostic radioisotopes may be the current focus in our local market, at HB Biotechnology we are actively looking beyond this, to the larger markets of radiopharmaceutical therapeutics.
[i] Prostate Specific Membrane Antigen – Positron Emission Tomography (PSMA-PET)
[ii] Metastatic Castrate Resistant Prostate Cancer (mCRPC)
[iii] Investigational New Drug Application (IND)
4 topics
6 stocks mentioned

