Anteris - with CEO Wayne Paterson
One of our local healthcare investments is Anteris, which is developing a new transcatheter valve for aortic stenosis. Their DurAVR device has been used in over 100 patients, and surgeons have been delighted with both the ease of use and results, which are measurably by both MRIs showing blood flow, and the patient’s immediate response after surgery.
The global heart valve market is divided into traditional surgical valve replacements, which require open heart surgery, and increasingly popular transcatheter replacements, which are minimally invasive and involve inserting the valve into a blood vessel and guiding it to the right place in the heart.
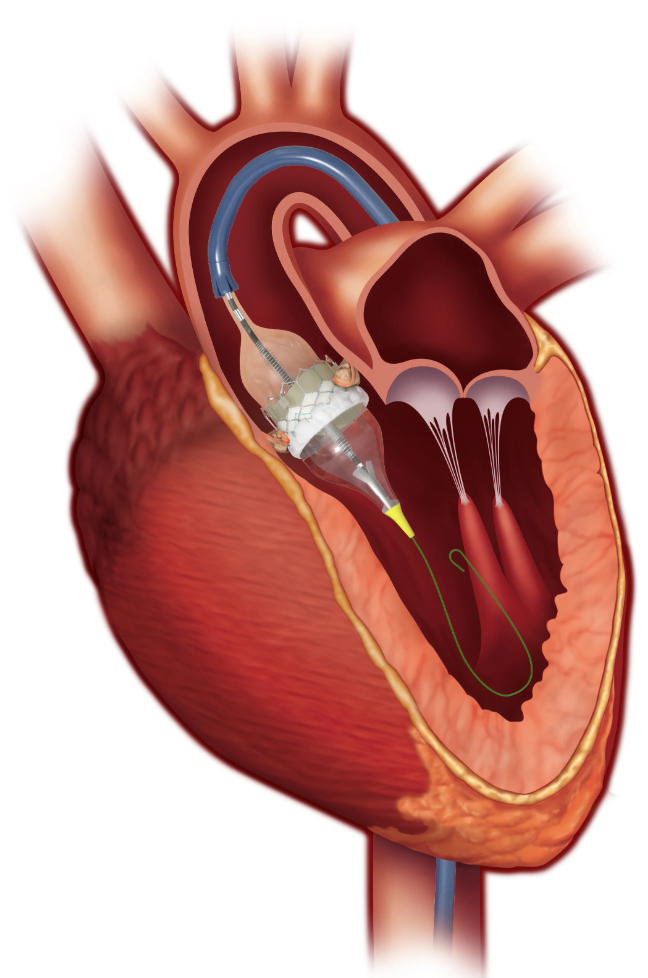
This was originally developed for older patients who can’t risk open heart surgery, but is increasingly common in younger patients too, and transcatheter valves are growing faster than the traditional surgical alternative.
Transcatheter valve replacements (TAVR), are growing at about 15% per annum, and have created immense value for the two key suppliers: Edwards (US$44 billion) and Medtronic ($109 billion). Edwards has greater market share at 60-70% with their valve requiring an easier, safer surgery, and Medtronic maintains ~30% share with perhaps a better valve, but with the unwelcome trade-off of a longer, more difficult and risky procedure. Abbott, Boston Scientific and others have minor shares.
Anteris uses a better material with less calcification (which is the main cause of failure in natural and replacement valves), but more importantly, has designed the valve to be biomimetic. So far, feedback is that it’s as easy to use as Edwards’s valves with better results, achieving laminar flow, less valve ruining turbulence, hence the favourable reviews from both surgeons and patients.
As transcatheter valves are used in younger and younger patients, and elderly patients are living longer, valve-in-valve procedures, ie giving a patient a second or third valve, are becoming more and more urgent. Anteris’s valve has shown particular promise here.
The company sold off ahead of a US-listing and US$88 million raise, but post raise, much of that overhang is gone.
The company is waiting for their pivotal trial result to be approved by the FDA, after which they will commence their pivotal study. Long term results are important, but the key metrics are measured after surgery and at the one year mark, and while follow up data is certainly required, approval doesn't need to wait, and the company will be paid for valves used in the trial.
Patients with aortic stenosis have a poor prognosis without surgery, so the demand is high, and the need for more durable valve with better flow dynamics is large.
This is not for the faint-hearted, but with the Nasdaq listing the company is cashed up, shares have significantly more liquidity, and our own conversations with KOLs have been universally enthusiastic. And with over 100 patients now living with Anteris’s valve, the clinical risks are lower than, say, small drug companies in a similar position.
The market for valves is about A$10 billion a year, with only two product families in the market. Anteris is trading at a market cap of ~US$150 million, with about half that in cash, so you can see the opportunity. We’re currently in a market where anything more than a year (or even a few months) is discounted almost entirely.
CEO Wayne Anderson describes it best, and I’m sure you’ll find the interview below interesting.
Michael Frazis
I thought it'd helpful now to just start with what transcatheter valves actually are and where they’re used. Can you give us a bit of background?
Wayne Paterson, Anteris
When we talk about valves, there are multiple valves in your body; several around the heart, the mitral valve, and the aortic valve. Valves are designed to allow blood to flow one way and not flow back in the wrong direction.
When those valves become dysfunctional, they either don't open wide enough and then create pressure a bit like a garden hose when you squeeze the nozzle and the diameter gets smaller.
There is a point where that pressure becomes dangerous or even fatal.
Or they have other problems where turbulent flow is created because of disruptions within the valve symmetry caused by calcification and irregularities.
The disease we treat is fatal if left untreated.
We didn't start life as a valve company, Anteris was a drug company.
When I was pursuing my board career, I joined the company as a director then became chairman.
I joined a NASDAQ board around the same time here in California.
It was clear to me at the time from where the company was, the share price publicly traded on the ASX, that the drug platform was not a viable investment thesis.
From my background, I can tell you that drug was not commercially viable. It was never going to be launched.
It requires about one billion USD to bring a new drug to market - that's the Merck number where I came from in the headquarters.
Your chance of success at phase two is about 5 -10%.
So, if you gave me a billion US dollars, our chance of return is 5%.
Not a good investment thesis.
But we did have one very key technology called ADAPT, which was collagen material and used in the surgical repair space.
I always describe this to physicians and others as a big company tech.
It's really high science, used in surgical repair. Which is great, but very commoditized. It's about a $50 million market.
That material is the building block for the market that we entered.
That's where we began the journey to look for an investment thesis beyond the drug platform, and beyond the $50 million market, which is not particularly attractive for the investment required to gain market share.
What else could this material be used for?
Our space was adjacent to the US$10 billion space we are in now. These valves are made from collagen, pretty much the same material we had.
We just didn't have the direction.
So we basically took a blank piece of paper to a very big space and asked physicians what they would like to see, then decided whether we could achieve that and create an investment thesis.
Overall, we wrote the product around an investment thesis.
We wrote the commercial plan, the launch plan, everything in about seven years.
Michael Frazis
How is the valve designed? Was that done in-house?
Wayne Paterson, Anteris
Yes, absolutely.
The thing about the disease, aortic stenosis, is that it's fatal if left untreated.
50% of patients are dead with severe disease in two years. No pharmacotherapy.
Michael Frazis
Why don't we start with how these patients present? What’s their patient journey typically like?
Wayne Paterson, Anteris
It's a weird dynamic. Obviously, it does afflict a lot of women, but more so men.
Australian men, US men, probably British men, don't tend to go to their doctors and talk about this a whole lot.
We are treating 15% of patients and that's still a $10 billion market. So, keep in mind, there's 85% of patients who are not diagnosed or treated, partly because they don't get diagnosed.
It’s a disease of symptoms.
The first thing you will notice, like a lot of cardiac diseases, is syncope or shortness of breath. Upon exertion, walking up the stairs, going to the bathroom, going shopping etc.
Patients will start to notice that exertion requires more breathing, and they have to sit and rest. The majority will put it down to old age, but this disease starts kicking in maybe in your 60s. There'll be other typical pains from a lack of oxygenation, such as chest pains.
But that's really the first one.
They'll present to the doctor. If they tell their doctor they have these symptoms, they will eventually narrow it down to a couple of key measurements.
Once you get over the symptom presentation, the physician then decides, OK, we're going to take a scan, look at a few things they'll bring it down to a measurement called the mean gradient.
That's how you calibrate this disease. It's a blood pressure measurement measured from within the heart. It's measured in millimetres of mercury.
Normal range is about 5 to 10 millimetres of mercury; a severe patient gets up to 40 or 50 millimetres of mercury.
You feel that pressure going up because the valve isn't functioning.
We've treated patients with 100 millimetres of mercury or more.
That's pretty much end-of-life kind of treatment, there's a spectrum of how this disease manifests from mild to severe, like all diseases.
If you get on it early enough and you start treating it, there's a couple of ways they could have treated this disease, but they must change the valve.
The interesting thing to me was it involved switching out a piece of anatomy.
That's how you fix this disease. The piece that we've been putting back in for 20 years does not look like the piece you were born with, and it doesn't behave like it.
There is our entry point because the current valves are not physiologically or anatomically correct, and don't give you a pre-disease curative outcome.
Our valve, DurAVR, is a completely different design.
We designed a completely different class of valve. All other valves fit in the bioprosthetic category. We fit in the biomimetic category.
It physiologically and anatomically emulates the valve in your body.
We own all the IP, so we own the class – no-one else can get into this class.
A big differentiator is that it gives you pre-disease outcomes, which is a big differentiator. It was really based on analysing the gap in the market and taking a different approach, as we couldn’t come out with another bioprosthetic, and, for example, make another valve like Edward’s.
Michael Frazis
Why don't we talk about what happens after completing tests and finding that you have high mean gradient. What are the existing treatment options?
Wayne Paterson, Anteris
The first steps are to look at your symptomology.
Firstly, is it debilitating for you? Do they need to take action today or can they take action tomorrow?
Increasingly, they're talking about taking action today.
Similarly to oncology, which is where I come from, where you can treat cancer from stage 1 to stage 4. Majority of treatments were in stage 4 when the patient is older and symptoms were high, and the patient was heading towards the end of their life.
The two things they could do is give you a surgical valve or a TAVR valve on a catheter, like we make.
A surgical valve requires having your chest opened up, called the thoracotomy.
They bypass your heart (stop it), go in and surgically insert the valve.
An 85-year-old cannot tolerate that without a high risk of death on the table, so they use a catheter-based valve instead.
No problems arise with that type of valve as the patient is nearing end of life.
These days, if your symptoms are debilitating, they might wait to see. Whereas if you are treated earlier, you’re going to have a better long-term outcome.
If your mean gradient is already 40 or 50 millimetres, they will treat you because you're already severe.
If it's 20 to 30, they might have look at your symptoms first and wait and see.
Every physician is different, but increasingly the age of treatment has come down a lot.
It used to be 83 years old, and now it's closer to 68 over the last five years. They're treating patients a lot younger, and talking about treating patients without symptoms.
They’re using studies to determine when the optimal time for treatment is.
Michael Frazis
So those existing transcatheter aortic valves (TAVR), it's basically a two-player market. There are some other competitors, but it's really Medtronic and Edwards.
Can you go through how those valves work and what the differentiators are?
Wayne Paterson, Anteris
When we look at the existing technologies, there are basically two.
This is what we learned when we had a blank piece of paper, we wrote our investment thesis around the product. The market in the States is roughly divided between 70% Edwards and 30% Medtronic
That's the question for me was why has that market share not changed?
How could that market share not over a decade?
And what we learned was that the barrier to adoption is in fact the delivery system. Self-expanding valves, like the Medtronic valve, are more challenging to use, in that it doesn't have a balloon, and relies on radial strength of the frame when it's inside the body.
Good operators know how to use it.
People who do lower volumes generally are a bit nervous about using that product. I've been in those procedures - they are much more challenging. They take longer than the Edward system, which is balloon-based.
Once the valve's in place, the catheter gets withdrawn and thrown out. But it's so critically important because you've got a physician on the end.
Now, balloon has a lot more control. Once you put the valve inside the body and the valve's crimped down, it's very thin going in.
The way it expands back up is that you inflate that balloon to a predetermined amount of atmospheres that you mathematically work out from a scan, get inside the patient, get the valve in place, expand the balloon. It pops the valve out. You then deflate the balloon, and the valve stays in and you pull out the catheter.
The interesting thing is that the haemodynamics are better with the self-expander than they are with the Edwards product. So the haemodynamics are better with Medtronic’s less popular valve. But it’s harder to use.
The Edwards valve takes about 25 minutes to implant, the same as our valve.
The Medtronic valve can take 45 minutes to one hour because it is more complex. And that's if everything goes well. So that's pretty much the difference to adoption and why one has significantly more market share. But the story is not just about the delivery system.
It's the price of entry. It's the barrier to adoption.
You talked about the other players, the reason that those other companies, Abbott and Boston, are not in the space, is because they're all self-expanding and difficult to use.
Only one company, Edwards, went with balloon expandable technology, I couldn't tell you what the rationale for that was.
That's why you only really see Medtronic, because nobody wants to really move or touch any other self-expanding product. They use Medtronic, but they have to small annuli, valve-in-valve female patients.
The balloon system is quite old. When we came with a blank piece of paper, I brought physicians down to Minneapolis from the biggest centers in the US, Cleveland Clinic, Columbia, chosen for their volume, podium presence and publication volumes.
These were people who we knew that in the future would help us launch this product if it was successful, and in Minneapolis we had a blank piece of paper, walked them through the Adapt product, and said, we've got a building block. If you had a new product, what does it look like?
They said, ‘it has to be balloon expandable’. Check. Thank you.
If I didn't do that, we wouldn't have a product or a company today.
And two, it has to be clinically better.
They wanted that delivery system, but they still wanted a better valve than what they were getting with Edwards.
And that’s why we landed on having to create a whole new class of valve. What they were asking for was, can you give us curative pre-disease mean gradients, 5 to 10 millimetres?
A bioprosthetic design couldn’t get to that pre-disease state.
So what we did was create a new class of valve called a biomimetic. Anatomically and physiologically looks like the valve in your body. And lo and behold, it's the first valve to give you five or 10 millimeters of mercury. It takes you into that curative zone.
Michael Frazis
So there's a real trade-off between ease of use (the more popular Edwards valve) and outcomes (Medtronic) in the existing competitor set.
We spoke to a surgeon who had done these procedures, and he said the Anteris valve was easy to use and had a strong effect. It didn't really have that trade off in the same way.
Wayne Paterson, Anteris
Yes, that was by design.
If we had come to market with the same issues, why would they use our product?
We were creating an investment thesis to see whether this company was going to exist or not. The only way that that was going to happen is if we had clinical differentiation.
To be commercially viable, you have to be clinically relevant.
If we weren't clinically relevant, particularly as a smaller company, no one's touching us. There's no investment thesis in the first place. We had to nail the sweet spot.
The only way we knew was for the doctors to tell us who do thousands of these things and decide whether we could actually achieve it.
We had a lot of sheer grit, tenacity, and incredibly talented people.
Minneapolis is the medtech center of the world. All the big company’s campuses are here. As a result, you have all the infrastructure, animal labs, cadaver labs, medtech engineers everywhere for the last 50 years.
It's the only place you could move this quickly to get to the point where we are now with a clinically superior product.
Michael Frazis
When you say your valve is 30% better, are you referring to that pressure measurement?
Wayne Paterson, Anteris
It's a bunch of things.
The 30% part is what the conventional metric for this disease is, mean gradient.
They calibrate this disease in every clinical paper, when you're diagnosed, they measure your mean pressure gradient.
That's how they know if you're severe or mild.
Take the SMART study for example, a recent head-to-head comparison between Medtronic and Edwards. One of the advantages in this space is the wealth of patient data, so we don’t have to guess how we perform.
We’ve treated over 100 patients, which is a large number for TAVR. All results are independently adjudicated by third-party core labs. We don’t handle the data ourselves. For context, in the SMART study, a 22-millimeter valve from Medtronic shows mean gradients around 20 mmHg off the table.
That’s consistent.
Any doctor will tell you that with a Sapien valve, you’re aiming for 5-10 mmHg to be considered curative.
In our patients, from number one through to 115, we’re consistently seeing mean gradients of 5-6 mmHg. You may have seen this data already, as doctors are presenting and discussing it. That’s the conventional metric. When we talk about being 30% better, it’s because we’re actually clinically curative right off the table.
That’s not a coincidence, our valve is fundamentally different.
It’s the only valve to produce laminar flow, something never achieved before. This is because the valve is physiologically correct. Laminar flow is key – it's directly linked to durability, mortality, heart failure and left ventricular hypertrophy.
We’ve measured this across other valves in various studies, and physicians globally are recognizing it.
And of course, the main gradients do matter. The lower that pressure is, obviously, the better it is for the patient. Our delivery system is also the easiest to use, but the laminar flow is what really matters.
Michael Frazis
To spell that out, laminar flow, has less turbulence.
Is that what we're talking about here?
Wayne Paterson, Anteris
Completely spot on.
Disrupted flow is known to degrade valves very quickly.
The biggest killer of a valve, as much as the tissue matters - and we've got the gold standard with ADAPT - the biggest killer of a valve is turbulent flow.
The interesting thing is that the current market leader has worse flow displacement, flow reversal, than an untreated, highly stenotic valve. And we've measured that in patients under the 3D, 4D MRI.
We now know why those particular valves fail quicker. There is no one with 10 years of durability data, but we do know that some of these valves are failing in two years.
And the ones with the highest amount of turbulence, which we can measure in patients on 40 MRI, have the quickest rate of degradation. It's very clear what that linkage is.
Whereas we have normal to near normal laminar flow, like very, very little, if any turbulence. And measure that flow as you can see it on the MRI. It's very easy to see in patients.
I'm not sure whether you saw that in Sydney valves a few weeks back, it's huge. Physicians have not been able to achieve that before.
Michael Frazis
Got it. So why don't we zoom in on the valve itself?
How is it achieving that laminar flow?
Could you also talk about the material and the advantages of that as well?
Wayne Paterson, Anteris
I think it goes back to when we were looking into the company and said, OK, we've got rid of the drug platform. What have we got left? Is there an investment thesis?
The Adapt product, the Adaptive Technology. There's a portfolio of Adapt products that we used in surgical repair. There are 55,000 patients with Adapt inside of them globally, which comes from Australia. And so that was a starting point.
The first thing is tissue. Why is it different?
The Edwards valve, for example, is also made from bovine collagen, the same as ours. They treat theirs one way. We treat ours a certain way as well. The big difference starting with the tissue and the differentiation of the valve, our tissue was designed by Professor Needling in Australia about 20 years ago, to be acellular, meaning there was no remnant DNA.
So remember, it's coming from another animal. It's going to go in your body. Every other tissue out there is decellularized, which means there are remnants of DNA in there. Very, very small whether it's in the surgical repair space for other companies or whether it's in fact an Edwards valve or a Medtronic valve, which is porcine.
Your body responds to that DNA naturally. It's a foreign matter over time. It's very small, but your body will have an inflammatory response to that DNA. And that's the precursor to calcification.
This is a disease of calcium. So suddenly these valves are, over time, having that calcification problem because of the DNA. Our material is different: acellular, meaning zero DNA versus some DNA.
That's the first point, the tissue.
It’s in 55,000 patients, it's the gold standard, but it's the price of entry. The tissue better be that good or you're not playing in this space. And physicians expect it. The valve design was our aha moment. That's the critical link. And I don't know why someone hadn't jumped on this before. The valves that are out there, be they surgical or TAVR / catheter based, are all in a certain class called bioprosthetic.
Bioprosthetic is three pieces of tissue sewn into a frame, three flat pieces of tissue with about 800 sutures.
Now 800 sutures means 800 holes, by the way. You have a problem with the integrity of the valve. The valve in your body does not look like that. The valve in your body looks like our valve. When we had the blank piece of paper, we had to be clinically better, in other words, disregarding the delivery system for a minute, how are we going to improve those mean gradients, those blood pressure measurements?
A bioprosthetic valve doesn't work like the valve in your body. And that's the bit I couldn't connect to. And we said, OK, doctors want this. We've got a blank piece of paper.
A company's future depends on it or not. And we played around with the bioprosthetic design like everybody else. They're all bioprosthetic valves. And we couldn't get 5 to 10 millimeters of mercury. And finally, one of the very smart PhD engineers that we're lucky enough to have in our company, Jason, said, let's change the design. And no one had really thought about that.
And so suddenly, we created the first single-piece 3D moulded valve that looks like the one in your body.
The one in your body should look like two hands praying and ours does and that gives you a bigger co-optation zone so you get a bigger opening and a lower mean gradient and more direct flow.
All the other valves hinge at the top like a door, all of them have a bioprosthetic dome at the top, so they narrow at the opening. so you get higher pressures and they cause turbulence because they're not they don't work physiologically like a valve in your body.
So the fundamental difference is that valve design, and we just took a completely different approach.
To add to this, the single piece gives you much more structural integrity and durability as well. Perfect laminar flow, of course, gives you much more durability. But it's the patient outcomes that are most impressive. I mean, we're getting pre-disease curative mean gradients, five to 10 millimeters of mercury.
We're reducing that left ventricle. Because of that flow being going smoothly, the problem with disrupted or turbulent flow creates back pressure. Your heart has to push against your left ventricle.
The more pressure, the bigger your ventricle gets. And that leads to heart failure.
When you've got normal flow, like ours, we're actually reducing the damage that's already been done on the ventricle. And we've measured that by about 30% over six months. So disease modification is occurring with this product in a way it doesn't happen with the others.
Michael Frazis
Can we go through the clinical data then, the trials you've done so far and any follow-up you've had with patients?
Wayne Paterson, Anteris
I've lived by data my whole career.
Big studies, 5 - 10,000 patients in cancer and other areas. But what I really like about this is I've had a lot more proximity to the patient.
The data we have is pristine.
There is the interactions that you notice with patients. I'll come back to that. So, 110 patients across Europe and the US. We've had physicians from Cleveland Clinic, from Columbia, a lot of the big centers have used our product.
We've got a physician from Australia, Carl Poon from Prince Charles. He's done an operation. There's an Australian physician over at Cleveland Clinic. He's done quite a few as well. So we look for the Aussie connections wherever we can find them. And there's a lot of involvement here from the Aussie side, of course. But the data is then adjudicated. We've done what's called an early feasibility study, which is just the normal process of getting through this.
You go from bench to animals to cadavers.
Then you move from cadavers to your small human studies, and of course your bigger studies. And we've done an FDA EFS study here in the US as well. And that's when it gets very serious, right? You're under scrutiny, you can't touch the data, you can't go in the operations.
It's all done by third party, so it's obviously clean data. And so those studies have amounted in total to, I think, about nine cohorts we're at the moment.
Nine different groups of patients over a period of three and a half years now between the European and the US EFS. You see the results get slightly better from the first patient to 110. And that's because the physicians get a little bit dialled in.
We've made a few tweaks on delivery as you're supposed to during this process. And three weeks ago, we did 20 patients in five days. That is commercial speed, 25 minutes.
It was crazy how much we got through. Now the data is consistently putting our patients, whether they start with 40 millimetres of mercury, we've had some of those.
We've had patients who have had 120 millimeters of mercury, which is off the charts. We've had those. They all get back to five to 10 millimeters, every single one of them. So we've all got them back in the normal range. We've treated bicuspid, which is a particular type kind of morphology, very difficult to treat. I think we've probably treated 15 or 20 bicuspids.
That's pretty crazy stuff. When you do a big study, typically in med tech and pharma, you tend to look for the healthiest sick guys you can get into a study, right? Which means by exclusion criteria, you're looking to treat a disease, but you don't want the patient to have 10 other diseases that might make a mess of the study.
That's not what we've got with these first 100. We have had some of the messiest patients you'll ever see, and the physicians will tell you that. That would not be normally included in a study because we wanted to push every boundary we could.
We had confidence in the product after about the first 10, 15 patients.
And then we've got a bunch of valve-in-valve patients.
Valve-in-valve is where patients have had a first replacement valve. It's failed and they have to put a second one in.
It's a bit like salvage therapy.
They're very sick. You can't crack their chest, of course. They're too sick for that. And there's a lot of junk in there where that first valve has failed. So you've got to get a second valve in and try and get some result. You're never going to get a good result with valve-in-valve. They're always going to come out with the worst outcome than their first valve.
And we didn't actually go looking for these patients.
Hospitals in Europe and Canada came to us and said, look, we've got this sick patient. Can we use this on compassionate use?
We must go through a regulatory process because it's not registered. We did 10 patients so far, valve-in-valve, and one of them was a triple. So, one of them had a failed surgical valve, a failed Medtronic valve, and then they put in our valve. You don't ever see triples. They generally pass away. We've got a better mean gradient in all of those patients than when they got their first valve, which is crazy.
These are patients with no good solutions.
There's no dedicated solutions. Doctors don't have a good solution. And it's a growing phenomenon.
They think it's going to be about 20% of the market or more by 2028, because we only started treating younger patients in 2019. Before that, they died. So now you're getting to the five or six year mark where these valves are now failing, which is a $2 or $3 billion market by itself.
And we are definitely the only game in town for valve-in-valve.
For the same reasons in untreated patients, right, is that valve design means they are getting better results than with their first valve, when they should be much, much worse and getting a much worse outcome.
That's the patient population we're treating, with very, very challenging morphologies and so on.
A lot of really difficult patients are getting great outcomes across the board.
The data is then measured up with external labs. They do the scans.
They read all the data. We're not allowed to just submit data. Then there are patients who I've met and I've seen.
I'm actually in a lot of these procedures. I'll scrub in, go in with the physicians. It's always a privilege, obviously, to do that. I'll see the patients before they go in.
With this particular disease state, you can really tell a patient is sick with a cardiac disease because their pallor is very grey. They have no energy. I mean, they look very sick. Some of them look close to death and some of them are close to death.
I interviewed one lady not so long ago and she was hitting the three-year mark. She was in her 80s. When we treated her, she looked terrible like a lot of patients. When I interviewed her, she looked 20 years younger and even the physicians were commenting on that.
Obviously, the product works really, really well.
But if you get a Sapien for example at three years again, you're not healthy.
So it's not like you come out of this with existing valves and they’ll get symptom relief.
They'll get some mean gradient drop no matter what product you put in, but then in two or three years again, they're looking pretty sick. They don't look good. Even if they live for the next six years. So, there's a whole bunch of things going on with these patients that even the physicians will tell you puts this product head and shoulders above probably because of the normal physiology.
It's very interesting.
The data supports it anyway. We have just the best data no matter which way you cut it consistently. But when you look, when you eyeball these patients, and physicians do often assess patients just visually, their power, their energy levels, the patients look amazing two, three years out, which they probably typically shouldn't with this disease.
A lot of these patients are older, so they have comorbidities, they have other diseases that go along with it, it's not just the cardiac disease in your 80s.
It's pretty encouraging for the future and doctors, and I mean, very high level, very famous doctors and big centers are all in behind this product for those reasons. It's just giving better outcomes.
Michael Frazis
That's been consistent feedback from the surgeons I've spoken to.
Can you help us do a bit of market sizing here? How many operations are there?
What's the existing opportunity? Is the market growing? How big is valve-in-valve?
You mentioned two to three billion.
Wayne Paterson, Anteris
Of course.
The interesting thing is that I come from an industry where you look at incidents and prevalence when you size the market before you launch anything.
You get a feel for the disease state. When I was at Merck, you go in three, four, five years before launch and you get the market ready for this particular product.
There'll always be competition.
When I looked at this market, the incidents prevalence data said to me that there was a US$30 or $40 billion market here. I couldn't correlate why the consensus on the street was it was $10 billion by 2020 US dollars, and that no one really had a model for it either, which was a little bit different for me.
Then I understood that we're only treating 15% of patients. So therefore, 85% were not treated. Everyone was fine with the fact that 10 billion is a big number. The truth is somewhere in between. And this excludes China, who by itself is probably a $60 to $80 billion market if you treated all of it. There's about 2 million aortic stenosis patients above 65 there.
If we go back into the US market, there’s about 175,000 cases per year.
What I love about this condition is that it's very compact to a number of medical centers. The top 60 to 80 centers in the US control most of the volume and certainly influence the rest.
In the past I've launched drugs with 5,000-6,000 reps around the world on day one.
When you size the market, you've only got this concentrated market.
And it's not a mature market, right? It's a growth market.
And they are already starting to treat patients with our valve.
The market leader has 70% market share in the United States, and we're 30% clinically superior and as easy to use as that product. All the big centers know us, they're behind us and they're looking for alternatives.
So in a $10 billion revenue market, if they have 70% market share, and by 2028 we get 10%, it's a billion US dollars.
Now, the centers we're going to launch, we can cover with an additional 30 to 40 headcount.
I think a lot of people think a launch is a massive undertaking. It can be, certainly in pharma, but that's not what this market is.
And we're not going to try and launch around the world on day one. Even we have European approval and everywhere else, we can obviously go stepwise. We can be cash flow positive in the first nine months post launch, just in the US.
Some folks will say to you, we can get 10%, 20%, 30% possible penetration.
But what we do know, is if you live long enough, it's 100%. All of these valves will fail.
When we started this journey about seven years ago with a blank piece of paper, companies like Edwards would say, our valve is a valve for life. And there was no data to support that. Everyone just assumed it was correct, because they were putting them in 85-year-olds who were dead in three years. So of course it was. No problems really pop up in three years.
The key thing is to choose your first valve wisely.
No one ever goes out and says “valve for life” any more.
If you start with an Edwards valve, you might then pick another valve like Medtronic to go for your valve-in-valve. They all acknowledge now that valves fail and that you will have to pick a second valve.
So now - choose your first valve wisely. That secondary market could be 30%, 40%, 50%.
Physicians and people who are particularly skilled at valve-in-valve will agree.
Anita Raskar, for example, in Chicago, will say we’re facing a tsunami of failed valves.
Now I can tell you unequivocally the only game in town for valve-in-valve is DurAVR.
And every doctor will tell you that just because of the results we've got today.
Michael Frazis
Amazing. How much do these procedures cost? What's the cost for a valve today?
Wayne Paterson, Anteris
There's different aspects and it's important to understand the health care systems around the world.
I've had the good fortune in my career to manage pretty much every health care system in the world as president of Europe, head of emerging markets, CEO of Japan for Merck at one point, and so on.
Now, there is essentially one kind of health care system, which is a cost-based model like Australia, Canada, and Europe. And then you have the US model, which is a profit-based model. And you'll see some of the data as to why these health care costs are so very, very different.
In the US, the sticker price for a conventional TVAR today is about $31,000 to $33,000 US dollars. That's not the procedure, that's the unit cost. Now, under the reimbursement systems up here with CMS, the procedure can be up to $100,000 for the physician time. As a company, you don't have to worry about that. It's all covered by insurance. The unit price in Australia is different.
Australia negotiates in a different way with companies. I believe it's more like $15,000 per unit down there. Either way, the cost of goods is a fraction of these numbers, particularly at scale. So there is good margin on these products.
But they are life-saving devices as well, so it's justifiable for the price is charged.
Do I see prices coming down with competition? It's possible. Doesn't happen in pharma. Prices go up usually, certainly in the States for drugs.
And I think we're definitely going to take a health economic style argument to the reimbursement, once we can prove mortality benefits and other benefits like flow and particularly left ventricular remodelling.
Disease modification, that comes with a price premium even in the pharma side. And the regulators understand that.
We're certainly looking to achieve that once we have the data to support it.
Michael Frazis
Got it.
What's the pathway from here to getting full approval in the United States?
Wayne Paterson, Anteris
We're actually at the final step now, which is the pivotal study. We've been negotiating that with the FDA.
You go through the processes we talked through before. You have your early feasibility studies in Europe and in the US. The FDA says, fine, we like the product. We like the safety. A bunch of physicians lobby for you as well. You have to have that.
I was down on the FDA campus in Washington DC about a month ago physically with our review team, the TAVR review team, with three professors, who are very well known to the FDA, finalizing the final steps.
Now, we have said publicly that we would make our submission for our IDE in Q1. We did hit that timeline. And we're under the review process now.
You negotiate for a couple of years with your reviewers to make sure there's no surprises, that they get what they, that you take their advice on biostats and numbers.
Where we landed was a randomized study.
That means we’ll do one of the first real head-to-head studies, half of them will be DurAVR, half of them will be standard of care, Edwards and Medtronic, ain a 70% / 30% split, like the market.
The primary endpoint is non-inferiority. And what that means is that you just have to be as good as the other products in terms of all-cause mortality, which is a composite mix of disabling stroke, rehospitalization, and the normal stuff.
And then it’s really about safety profile. Of course, we've already got enough data of patients to already know where that's going to land. It's not like there's any surprises there. It's not like a drug where things can change. It's a physical device. Things don't change.
Our secondary endpoints are around hemodynamic superiority. We already know where we land with that. Things like the left ventricular remodelling, we're going to put that in the study as well. And then there's a valve-in-valve registry. So the valve-in-valve registry is basically just collecting patient data. It's under the same umbrella, but it's not the head-to-head, we don't have to do it.
So it's pretty straightforward.
A collection of maybe 100 patients collects that data.
They'll approve us for that label.
It's a global study. So it includes Europe. The FDA has allowed us to put European patients in to get that number up. But the Europeans have also allowed us to get a CE approval with their sites. So of course, we're bringing those on.
That's the first part. You negotiate the protocol. Once you've got enough data to give the FDA that they're satisfied with safety, efficacy, and so on.
And then you qualify your sites, the process we're in right now, there's obviously a cost factor that goes up a little bit at this point, which we've already taken on.
It's not like those costs are coming. We've already taken those costs where you must have people out in the field. And those people are qualifying sites, getting the contracts organized, getting the hospitals ready once the FDA approves your protocol to then go forward and start the enrolment process, across multiple sites.
Now, physicians are hugely enthusiastic for this product. That's a fact. And you may have experienced that, but certainly here in the States, the demand to enrol in this study is greater than the hospitals we can support, which means it will be a fairly quick study to go through.
Cleveland's the biggest cardiac center in the world. So, you can imagine the sheer volume that goes through there. And they've been on our advisory board since day one.
It's about the clinical outcomes. People are excited and enthusiastic because they already know from the data that they want to give their patients this. There is a one-year review with the FDA.
Michael Frazis
After one year for each patient?
Wayne Paterson, Anteris
The whole cohort.
You basically accumulate the whole lot, give it to the FDA. They follow up. You follow up at a year.
That's an important year because you already know what the data is. It's not like we're not allowed to control the data, but I certainly know from our patients. I get texts all the time, just did a patient, got five millimetres of mercury, even if I'm not in the case.
So of course, we'll know when we land.
This is not a product where you go through a study and you get a different result like a drug. There's been a few examples recently in Australia where a phase three study obviously went the wrong way.
This is not what you have in MedTech. It's not a Phase Three. I've done a lot of those. It's a pivotal study. The physical product doesn't change. There's no binary outcomes here. And that one year is when you're kind of putting some folks out in the field, you're building inventory.
Most important, you've got to have stock to sell. And we have a facility here in Minneapolis, a factory, where we make valves we need for the trial. And it's a decent sized factory. It's all FDA qualified, know, engineering and all these things.
We can support commercial sales for at least the first two years with the facility today. And that's really important because that was built into the plan years back. You can't just flick a switch when you get an FDA approval. You've got to plan that years out and that's where we are today. Some of the costs we incur are certainly around manufacturing.
And then if we decide things are looking positive in the first year, we'll obviously then think about whether we're cash flow positive, what investments we would need to make in more manufacturing to make sure we get more capacity going forward.
Those are the things we look at.
Now, Europe may come through earlier. There's no commitment either way on that one at the moment, and the CE process can be a little bit quicker. So we might see a European approval, in fact, before we see US approval.
Michael Frazis
Right. And is that approval going to be based on that one-year mark?
Because obviously these valves can be in patients for many years.
Wayne Paterson, Anteris
Yes, but again, the critical point they look at is the acute phase.
The first thing that regulators look at is what do you look like when you get off the table?
Obviously, our product is superior by far on that front.
They do look at the long term, but no one's got long term data.
And that's the interesting part of that.There are some misnomers about who's got long term data durability. We now know why these valves fail. We know they are failing because the valve-in-valve market is growing.
We do have to do a 10 year follow up, but that doesn't affect our commercial approval.
There is a commitment from all companies to follow patients for 10 years where possible. It's not always easy to keep these patients coming back and they sometimes die of other things as well.
Michael Frazis
Got it. So, give us a rough timeline then for when that would be.
Wayne Paterson, Anteris
Yeah, so I think I've taken a conservative approach due to capital, you need to be sure you've got enough money to run a study.
We're looking around about Q4 2026 to be fully enrolled.
So we’ll come out the other side in Q4 2027, and pop outside of that with an approval early 2028.
Now, Europe could be up to a year earlier than that, we don't really know.
Valve-in-valve, also not really sure. Valve-in-valve is such a huge unmet medical need that may come out a bit quicker as well, because they need those products out there.
And that's a pretty small registration kind of approach. So, there are a few variables. I'm not going to commit to those at the moment until I get more clarity. But I'm just working on conservative, longest case scenarios here.
Michael Frazis
And I guess you can charge for these valves, can't you?
Wayne Paterson, Anteris
We can in the study, again a little bit different to a drug study, right?
We actually get US$25,000 in the States for every one of our units in the trial.
Obviously, it doesn't run the company, but it's still revenue. And the procedure is covered by insurance, under a group called CMS.
In a drug study, you have to pay for everyone else's drug and all your controls and whatever. Here, the hospital buys our product as if it was a commercial product.
If you're in the control arm or if you're in the randomized arm where you're going to get an Edwards or a Medtronic product, that's already covered in the system.
We don't pay for any of that.
So when the patient comes in, they’re going to get our product, an Edwards product or a Medtronic product, either way it's all covered. And our product is acquired through the hospital as if it was commercial for $25,000 per unit. So not quite at the market level in a study, but certainly beyond the cost of goods. Then there is the second part of the study.
The bit between approval and the final enrolment is interesting because you can have expanded access. What expanded access is, that hospitals who have been in the study and only hospitals have been in the study can use the device.
We've got a list of hospitals for this particular reason as well, and they’re able to continue to use the product at will for $25,000 on any patient they want before approval comes through.
A hospital that's not in the study cannot do that. But take Cleveland Clinic and some of these big centers, they're all going to be in the study. They can continue to use the product before approval comes through.
I don't have a good feel for the forecast on that yet, but certainly that's obviously something to look at. And technically, you could be picking up your first pseudo commercial revenues in Q1 of 2027.
Michael Frazis
Got it. While we're on financing, Anteris was obviously ASX listed and is dual listed in the United States.
Where does that leave you with cash and how have you found that process?
Wayne Paterson, Anteris
I've become really comfortable.
There's such different exchanges. I’ve sat on a NASDAQ board, I’ve sat on an ASX board.
You know, there's a tendency to understand that the pool of capital in New York is obviously bigger and it is. What I do find a little bit different, we've got some great healthcare funds in Australia that I really appreciate, but not a lot of them, no real experts (MF note – thanks lol).
There’s a tendency to look at all healthcare companies the same, I often find the distinction between MedTech and Pharma with some of the more generalist funds is not understood. Therefore, they don't necessarily understand the risks of a Phase Three trial versus a Pivotal.
You might get tied up in that brush and that leads to a more diminished market cap than you'd like to see. And we can talk about the current environment in a minute.
Whereas in New York, you have like one of our biggest investors, Perceptive, is a dedicated MedTech specialized fund. They know Edwards, they know Medtronic.
They know the CEOs of those companies like they know me, they know how to place the product in the market. The analysts are also really important. So the analysts that cover us, Barclays, Cowen, those two other analysts up here that cover us, none of this is paid research.
Wall Street looks at that stuff. And so people like Josh Jenning and Matt Mitzik, the Barclays and Cowan's analysts are known dedicated TAVR experts and have been for decades.
People read their notes, they know the CEOs of these other companies.
They take your product. They know how to place it. So if you read their research, you get a very clear view. So that's one of the key differences. There are pros and cons, of course. We moved primary listing to the US. And literally, a couple of months after listing, the world falls apart because of issues around tariffs and market uncertainty. It’s just one of the things.
The markets are spring loaded up here. That's the critical part. The one thing you don't have up here is I was in New York last week. The banks are not panicking about the market or about your stock.
40 other small caps in their portfolio are down 70%, and so, everyone realizes that it's just a macro issue.
I think the ASX is a little bit more cushioned from what's happening in the US. But when you see the price of gold hitting a record high like it did today at $3,400 a troy ounce US dollars, and the VIX is off the charts, then you know it's a macro issue. And I note today there's no rhyme or reason. A few weeks back, we were up 20% on one day.
No rhyme or reason.
Last week, we were up 18% on one day and the markets were up 0.6%. Today, we're up 10% and the markets were down 2.5%. And then on other days, we go down 15% and they go up 3%. So there's no rhyme or reason. Today, surprised me. The markets were red. We went up 10%. There's so much uncertainty.
Well, what it does tell you is the market doesn't hate us, right? Because if you're not in good shape, they're not touching you at all.
There's a lot of nuances, a lot of different players, a lot of experts in the TVAR space, which certainly helps you when you're trying to get credibility in a tough market, when you've got people at that level saying, this product is the real deal for these reasons and can then quote the data, it certainly helps you.
They're just very different markets. think the, I always have an affection obviously for the ASX We have a lot of retail investors and institutions, but this company before I even got here, some of those retail investors have still carried through.
Some people I've really come to have friendships with, I really appreciate it.
I communicate with them a lot. Some folks are Aussie characters and I just take great delight in having interactions with them. I do enjoy that level of contact. And our biggest shareholder is L1 out of Melbourne and Raffy and his team have been with us for quite a number of years.
There's a physician in their team, Andrew Lin, who certainly has that ability to analyse the data as well. So it's just a bit different mix, but I really am appreciative of the folks we've got on the ASX, because some of them I've just known now for six, seven years.
Michael Frazis
Got it. And how much cash do you have now after that raise?
Wayne Paterson, Anteris
Yes, we raised around 85 million US dollars and I'm burning at the rate I said I would, we're meeting guidance.
I think we burnt well, we burned about 20m in the first quarter and are on track to do roughly the same in this quarter.
Michael Frazis
Yep. Got it. And what's the mood over there in terms of the FDA, the firings, how are your friends in industry seeing things?
Wayne Paterson
We're very close to this - as close as I've ever been to the FDA in my 30 years in global health care, which is great as a small company.
Big companies get good access like when I was at Merck.
Of course, we're on the campus in the in D.C. a few weeks back and the firings had just started.
Now, I think it's definitely happening a lot on the pharma side versus the device side. But, you know, there's a lot of indiscriminate federal job downsizing, so there's a lot of uncertainty with federal workers.
The FDA is definitely caught up in that. Our review team who are TVAR experts are seemingly untouched at the moment because you take them out, who's going to review TAVRs and valve products and whatever.
You've got that kind of that expertise, but I think everyone's, you know, a little on edge, not only with what's happening with the federal workers, but also with what's happening with the federal government.
The tariff discussion has just put everything into a spiral and we saw the markets collapse as soon as that came out.
Michael Frazis
Manufacturing in Minneapolis, that shouldn't be such an issue for you.
Wayne Paterson, Anteris
Well I made a decision,. Here's the thing, the other valve companies, Edwards and Metronic, their valves are very labour intensive.
Now, ours are less labour intensive because of the single piece 3D design, right?
So it can take about twenty four hours to make an Edwards valve, and about six hours to make one of ours. So that will eventually run down to the bottom line as we get into commercial scale. But a lot of that stuff is done outside the US for the reasons of labour costs. And we're adamant we're going to do it all here.
As it turns out, it's 100% done in Minneapolis because it's a medtech hub, also has contract manufacturing for medical products.
Our catheters, of course, are made by a third party literally next door to us. But all the valve manufacturing, the tissue is made in Australia. So that's done in Perth. We have a factory there with about 20 folks. And that's going to stay there. But fortunately, due to the history between the US and Australia, we have a 10% tariff with Australia.
I'm not too worried, but everything else is made in the USA. We have zero, zero exposure to tariffs at this point for what it's worth moving forward. Who knows?
Michael Frazis
Got it. This may be a harder question.
I guess a challenge when you're looking at a company like Anteris is trying to understand how the rest of the field is moving.
Ultimately, you're chasing a moving target, in two years time, there might be other products in the market. There might be drugs available. Obviously, Medtronic, Abbott, Edwards, they're all going to be innovating as well.
What are you seeing on the innovation front from other companies that we should be aware of?
Wayne Paterson, Anteris
It's absolutely my job to understand the competitive landscape.
Like it was at Merck, like it was at Roche, and it certainly is at Anteris.
If we create an investment thesis around a product that has clinical benefit, you've got to understand the competitive landscape. The last thing I want to do is raise capital for eight years, have a product come out the other side, and there's a competitor product that's better from day one. That's not a good feeling. I would deserve to be shot for that.
You have a whole function in your company where you're looking at competitive and strategic landscape.
On the back of that, I do meet regularly with Edwards, Medtronic, Abbott, Boston, and J &J, meeting regularly with the leadership teams. Or they seek us out and have done for a long time. And we have talked about deals.
We're not strangers to each other. Medtronics is in my backyard here. I have dinner and I have meals with these people. And we talk about a lot of things, and certainly have talked deals as well. But the biggest source of your competitive intel is those companies' business development teams, because that's who our teams interact with.
The J& guys scan globally. They say we're going into valves, they look at every valve from Germany to Africa to wherever. And so the BD teams, like I had at Merck, are global. You're sourcing product everywhere. And when they come to us and they'll look at our valve data and will say, well, this is the only game in town or it's the best product in the world.
Do you get a lot of that feedback? Do I take that with a grain of salt? Yes. If I relied on that, I wouldn't be doing my job as well. So we use industry, use the bankers. If there's competing technologies,
They're talking to the funds. They're trying to raise money in New York. So I know who's out there. I know who talks to my bankers and then all the funds that we talk to. And I don't just talk to our investors.
I talk to a wide mix of funds who aren't invested in us and constantly have these discussions, you know who's talking around.
Then there's the physicians - we work with physicians who are well known to be the best in their jobs. As you know, then you've seen our list of people. Of course, other companies who have competing technologies or hope they have competing technologies try and get those guys to work on advisory boards the way we did.
They'll tell me, oh, this company popped up. A lot of them are in Minnesota because it's a med tech hub. So when these companies start off and they generally start here, or Geneva or Israel, they tend to be in those three places. So we put all of those bits of information together.
We scan all the research journals, all the data that comes out of that. And yeah, there are people. But the biggest thing that keeps you safe is once you've locked in IP like we did.
Remember, there was a gap in the market. We were the competing technology. We came out with innovation that Edwards didn't do for 10 years. And then you know, why would it try to buy me?
Once we got in there, we locked it in with IP.
I had IP lawyers in the room from day one with those physicians valve, valve frame, suturing patterns, the catheters, the delivery systems. The packaging box has some utility because you can use it in the OR to help prepare the valve.
We patented that. There were just dozens of granted patents for our products. So we now own this class of valve. It's a new class of valve, it gives you pre-disease results, and that's why they’d want it, because it's a new class, a different design. You can't copy it, because we have all the IP.
You can't copy our delivery system. If someone's creating something that looks and works differently to the valve in your body, because that's what we did. We created the biomimetic valve physiologically anatomically correct.
I don't personally know what you can do beyond that to get a better mean gradient than a curative result. We give you a pre-disease mean gradient. So if someone got a technologist going to give you better than a pre-disease mean gradient, there's no such thing. We're going to give you better than normal laminar flow, no such thing. It's normal or it isn't kind of stuff. We've kind of landed in that spot.
Any physicians will tell you, and the banks will say, it's not my opinion on whether this product is a revolutionary breakthrough.
We have shifted the discussion, right? We are that guy on the podium out front and that's why the companies talk to me and that's why these physicians work with us with the biggest names in the industry.
I think the big one is your IP protection.
If you don't get that right, well, yeah, you're going to get blown up in the middle. And so it's a matter of clinical outcomes and a bunch of other things.
Pharmacotherapy you mentioned. Now, I do want to address that because that's where I come from, pharma. I understand pharmacotherapies. This is the disease where the calcium builds up on your leaflets predominantly to a point where the leaflets become heavy and they close up the opening to your aorta, which restricts the opening, which raises the pressure, and then you get turbulent flow because of all the disruptions in there, all the irregularities.
The only way you can treat that with a drug - and this is why there's no drug for this disease - is to find a drug that will actually burn that calcium out of a leaflet.
Now, if you burn the calcium out of a leaflet with a pharmacotherapy, one, you've got to get it delivered in the right place. The drug has got to land on those leaflets, and how you get from the liver or the kidneys to the leaflet, I have no idea. It would be a bit like chemotherapy, where you've got to blast the body.
Two, if you're burning calcium off leaflets, you're to be burning the leaflets. So there's no imaginable chemistry that I can think of, and I grew up in biotech oncology.
Roche, the biggest oncology company in the world with all of this really cool biotech tumor-based targeted therapy stuff. So I understand targeted therapies. I grew up in it. And there is no conceivable way that I can see that you can target calcium on a leaflet with a drug today.
There is some recent research from some existing classes of drugs used for other diseases which they believe could slow down the calcium cascade, but it can't reverse the disease. And that's very early data. It wasn't core lab adjudicated.
I've read this data. We all know about it. Core lab adjudicated means the third party has looked at the data as opposed to someone in the lab doing it and saying, look what I got. There is a difference. But some of that data, if I'm generous with it, I would say, if you start taking that drug in your 20s, you might avoid stenosis or you might slow it down, but you cannot reverse it.
And so where we are today, and even to prove that point, you're years away. That's a big study, thousands of patients, lots of years. So it's a long time before that becomes viable.
Even so, there is no drug that I can see, understanding the chemistry of this. Today you've got stenosis. I can either give you a valve or give you a drug. It's just not possible as we stand here today.
And on the backside of getting innovation from the other companies, don't forget that if for some reason one of these companies developed a new product tomorrow, they are seven or eight years behind us.
They can’t just snap their fingers and come to market. They've got to go through exactly the same process, FDA, first studies, pivotal studies, and so on. And we watch all the patent filings as well. We monitor the patent office to see if anyone's filed anything new or something we don't know about, nothing.
We just did one last month, actually. So these are all the ways we keep track of things.
Michael Frazis
Okay. Helpful.
In terms of strategy, I mean, how you think about financing, partnering, M&A, etc?
Wayne Paterson, Anteris
Sure. I mentioned earlier, we meet fairly regularly with the other companies, at the senior management level.
We do have these discussions. I grew up, again, at a time in Pharma where Roche acquired Genentech, piece by piece and incrementally. And Roche is the number two drug company in the world. It's massive.
That's where I worked originally. Started in China. And both companies did really, really well. Genentech did well, if you know the story, and Roche did well.
Eventually Roche acquired them not so long ago and became the biggest oncology company in the world. So those kind of co-development, co-commercialization deals work.
At Merck, I did a co-commercialization with Bristol Myers Group BMS on a cancer drug. We both did well out of that. MedTech has maybe a different approach. My view on this, and I talk to companies, I will absolutely entertain a co-promotion deal, like a partnership that says, you guys can take Europe, we'll take North America, we'll use some resources, so on.
It's a very compact market, so we can do that. I contemplate a co-development deal, but we don't really need it at this point. We're into our pivotal. So there would want to be some big gain for us on the other side, because I wouldn't give up royalties for that. I would not contemplate an acquisition deal at the moment.
We have had offers. And the simple fact is that the valuation curve, I use a net present value.
I use an NPV. It's not blind hope or dumb ambition when I value our company. It's in their present value model I used at Merck to launch multi-billion dollar drugs. So it's not a Mickey Mouse model. And it lands at a very different number to our market cap. And one could argue that I've got a very conservative NPV.
But if you know NPVs and you know there's a TAM here of $10 billion and you're superior to the market leader, it doesn't take a great leap of faith to work out where that market cap ultimately will head.
Isaac Newton is right, gravity will take over at some point. And therefore, doing an acquisition at 500 million or a billion doesn't make sense when three years later, you might have a market cap of five billion. And it all sounds a little unhinged, but the math is your friend in this scenario, regardless of where the markets are at today. And there's a general skepticism around a development-based company.
Some folks may be not even understand that a pharma company and a medtech company have different risks. Like our level of risk is down dramatically.
And think about this, the NPV will always come true. But because of market dynamics, because of a bunch of stuff, we're at the most de-risked point in our lifecycle with 100 plus patients.
And look at where we're trading at, let's take the ASX. Two and a half years ago, I presented those first patients. We had 15 and we had a share price of $28. It went from 15 to 28 literally within that week when I presented that patient data.
And yet now we've got a lot less risk, a lot more money in the bank, and we're staring down the barrel of our registration study.
There's no rhyme, no reason.
People often try and conflate a share price with a problem in the company. And a lot of the ASX folks ask, well, what's the problem? And I'm like, well, firstly, if there was a problem, I'm legally obligated to tell the markets.
You can just take that off your mind for a minute. If there was some major drama here, I have to come to the markets to tell you, otherwise I'm going to end up in an orange jumpsuit.
People have got to understand that if they haven't heard something bad, then there's probably nothing bad.
Secondly, the risk levels for our company are far lower than they were two years ago, three years ago, and so on, and far lower than a typical phase three study for obvious reasons in the market. We have one recently fail in Australia. And that's kind of spooked people a little. And it's just a binary outcome in a phase three, which is not the case for Pivotal with the medtech.
I think there's a little bit of frustration. But also, you get kind of scooped up into people combining medtech and pharma together when they're just very different animals from a risk point of view and an investment thesis.
Michael Frazis
Definitely. So, what can we expect of the rest of the year?
Wayne Paterson, Anteris
This is a big year. Obviously a very big year.
Beyond the NASDAQ and those things that happened along the way here, which in itself is actually quite a milestone.
We just crossed over 100 patients.
We will, in line with our public statements, expect to get our FDA approval to start the IDE. And that's coming. That submission has been made. It's under review. There's a statutory time frame. We know what it looks like.
We'll get out the other side of that and commence that first patient in around about July-ish in the summer up here.
That's a huge milestone. There's a couple more in the background that may or may not come to fruition, so we won't get too much into that. But it is really, by far, will be the biggest year for milestones for us in the company's history.
Beyond that, then our focus it to just finish the study, get the approval, do the Europe thing, get the valve-in-valve indication, and so on.
Michael Frazis
Yeah, got it.
I thought it was interesting earlier when you said, that, pharmaceutical companies try and pick the healthiest patients in a trial, which obviously is a plague in early stage trials.
Because often an open level study will do better than, than, than what historical data would suggest. And then companies raise a lot of money, but ultimately the drug might go on to fail, you know.
Wayne Paterson, Anteris
We run studies with five, six thousand patients at Merck globally, and I've been involved in those.
I think you have a protocol that has exclusion criteria. I think to be fair, yes, you want to get an approval, but no, you won't do anything dangerous at a big drug company, right?
You definitely look for it, but you're trying to just pass out all the comorbidities and you're dealing always with sick populations like cancer or older patients. They're going to have 25 things wrong with them that could influence the outcome unfairly, right?
If your drug doesn't get approval for cancer because people died of diabetes along the way, these are the things you want to know.
You're looking for the disease that you're treating, check, they've got to have that. Then you're trying to rule out other things that might cause a high mortality rate unrelated to your drug.
We talk about the healthiest sick guys. And it's true for everything. And I don't have a problem with trying to just zero in on your product and trying to exclude any other diseases that will influence the data.
TAVR patients are older. They have a bunch of things often going wrong with them. And my point before is when you look at the TAVR studies where people got registration before, the exclusion criteria is big, right? And we didn't exclude those patients. We included patients that every physician who's worked with us will tell you would never have got into the other early TAVR studies. And the reason we did that was because we could be, #1, a bit more bold and a bit more confident when we were confident with our data.
We wanted to test the limits. If I'm going to go out there and say we're going to be 30% better, we better be.
And we better know that along the way, that we've got good flow and less side effects.
You really want to know that early on. So that's why we picked up patients with 120 millimetres of mercury on a mean grading. They would never make an early study of those patients. I was in the room with one of those one day. I was nervous. Patient looked incredible, left the hospital with 8 millimeters of mercury a day later. Pretty incredible study.
Michael Frazis
Yeah, well must be, I mean how does it feel working in the medical device phase rather than in pharmaceuticals?
Because in pharma you do have those days where the whole program can be cancelled or be an amazing success.
Wayne Paterson, Anteris
I’ve spent 30 years of bringing health care products around the world to people.
I mentioned the regions I had controlled before, I've got six doctors and five doctors in the family here in Minneapolis. My brother-in-law, one of my close friends for many years is an interventional cardiologist.
It's a part of my DNA. But I tell you, when I go and see patients or I hear doctors talk about our product and know that part of this came from Australia, part of it was designed here, you had your head in part of the development and you see those patients doing really, really well.
At this point in my career, that's a very, very good feeling. And I think it's important for the investment thesis. We're not just making widgets. Now, some companies can fail when they try to do that, and that's unfortunate for shareholders. We're not that company at the moment. So shareholders can feel really good. I know there's been some up and down times for everybody, but we've created a product. We did what we said we would.
We're creating better outcomes for patients than they had before in this fatal disease.
Ultimately the financial part of the thesis will catch up with the clinical side of it, so it's a very good feeling to hear doctors globally say, wow, this product is just so incredible.
The biggest names in the world on the podiums are saying that about our product that we've all been involved in.
It's a really, really good feeling.
And now the share price has just got to catch up with the rest of the good feelings and meet the market and everybody's expectations. I think we've met everyone's expectation on the science and development.
I think what's lagging, obviously, is the share price to catch up with those expectations.
Michael Frazis
Well, maybe that gap creates an opportunity.
Why don't we wrap up there? That was really fascinating. So, thank you so much for the time.
Wayne Paterson, Anteris
You bet.
Thanks, Michael. I love the way you're approaching it. Very matter of fact, but just great questions that allow me to kind of talk about the company.
2 stocks mentioned

