Biotech reawakens: policy clarity, breakthrough data and a surge in M&A
Sentiment toward pharmaceuticals—both emerging biotech and large-cap pharma—has shifted meaningfully since we last wrote at the end of FY25. Concerns around US tariff risk and “Most Favoured Nation” (MFN) pricing dominated headlines in April, but these issues have since been largely neutralised. As policy uncertainty has cleared, investors have returned their focus to fundamentals.
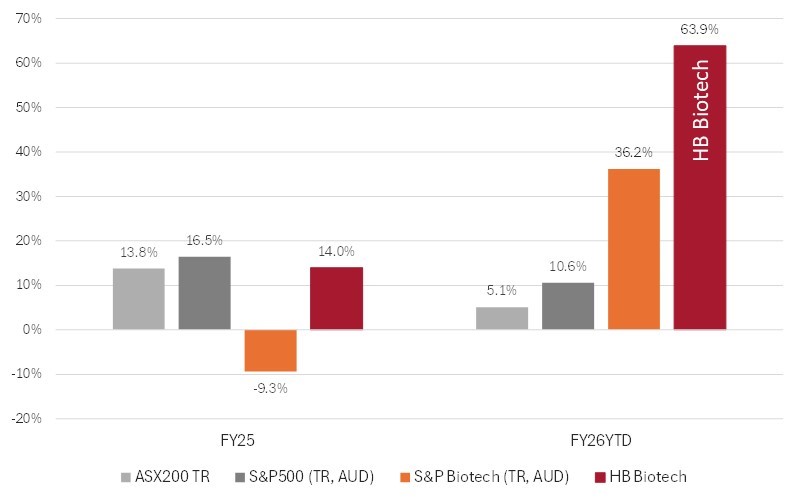
From 30 June to 31 October 2025, the S&P Biotech Index rose more than 30%. Over the same period, HB Biotechnology portfolios delivered a 63% return, driven by multiple outstanding clinical readouts and two strategic takeovers of portfolio companies.
Despite this global strength, the ASX healthcare sector has lagged (ASX300 Healthcare -14% FYTD), led by the ongoing weakness in ASX: CSL—once again underscoring the need for a global, diversified approach to healthcare investing.
Clearing Events: From Tariff Fear to Policy Clarity
In April 2025, the US administration fired a broadside at the pharmaceutical industry with threats of 250% tariffs and an MFN pricing regime that would align US prices to the lowest in developed markets. At the time, we concluded the practical impact would be limited for numerous structural reasons. Importantly, the current US administration left the door open for companies that were willing to expand US manufacturing footprints and participate in targeted price-reduction arrangements.
By mid-year, "ground-breaking ceremony" photo opportunities had become a staple of big pharma communications as companies accelerated US manufacturing commitments (in fact, prior to the April announcements, many already had existing plans to increase US manufacturing).

The turning point came on 30 September with Pfizer CEO Albert Bourla’s Oval Office announcement of a direct-to-consumer pricing arrangement. The deal met the administration’s political objectives while sparing Pfizer from material earnings impact.

Markets took this as the all-clear. Large-cap pharma stocks recorded their best single-day performance in decades, rising ~10% across the board.
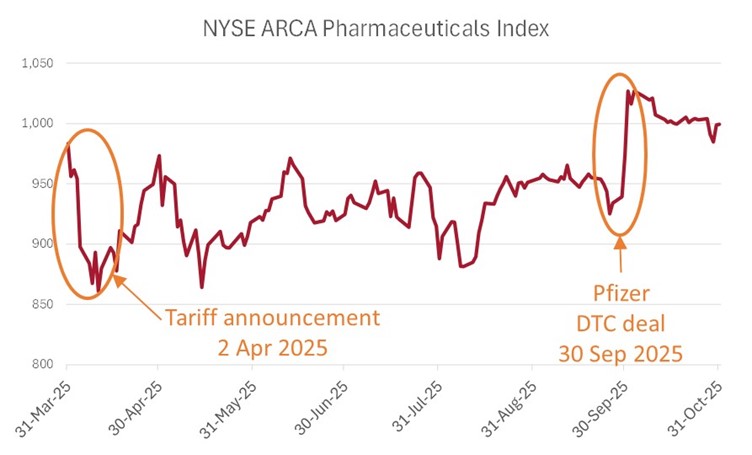
Many other large pharmaceutical companies have subsequently followed suit, including AstraZeneca, Merck, Eli Lilly and Bristol-Myers Squibb. Most recently Novo Nordisk and Lilly each cut deals to offer either cash-pay or Medicaid patients lower cost access to GLP-1 (weight loss) drugs in exchange for greater access and coverage.
Crucially, this overhang had been weighing not only on big pharma but also on small- and mid-cap biotech—the core of our investment universe. With the fog lifted, attention returned to clinical data.
Clinical Data Returns to Centre Stage
For several years, macro factors— rates, inflation, and Washington—have overshadowed fundamentals. With policy risks receding, the market is again rewarding true innovation.
The last six months has delivered a string of significant datasets that have been rewarded by the market, including many from HB Biotechnology portfolio companies. While incremental improvements can drive commercial success, very few therapies are genuinely practice-changing. One of our portfolio companies, in particular, produced such results:
Celcuity (NASDAQ: CELC)
In July 2025, Celcuity reported Phase 3 data for gedatolisib in advanced HR+/HER2- breast cancer. Patients lived 4× longer without disease progression (PFS 9.3 months vs 2 months / HR 0.24) compared with standard therapy—paired with fewer side effects than other drugs in its class. While not approved for marketing yet, key opinion leaders interviewed are of the opinion that these results merit a new standard of care for this cohort of breast cancer patients. Consequently, CELC shares have risen more than 6× since the day before the data were released – a clear illustration of the asymmetric returns available when innovation is genuinely transformative.
M&A Accelerates as the Patent Cliff Nears
Another major driver of the sector’s resurgence is the pickup in M&A. Large pharma faces a well-telegraphed loss-of-exclusivity (LoE) cliff, including:
- Keytruda – US$29.5bn 2024 sales, LoE 2028
- Eliquis – US$13.3bn, LoE 2028
- Humira – US$9bn, already off-patent
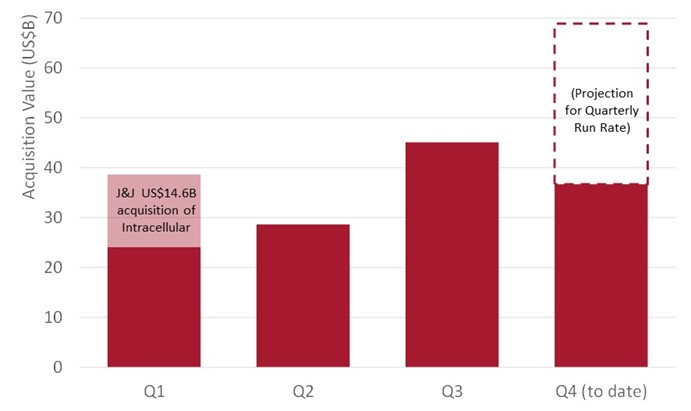
To fill looming revenue gaps, big pharma has returned forcefully to the acquisition market. The last few months saw the first bona fide bidding wars in years, including the high-profile battle between Pfizer and Novo Nordisk for Metsera (ultimately won by Pfizer for US$10bn).
For Novo, currently being one of the two leaders in the obesity market (albeit losing ground to Lilly) this move to acquire a pre-commercial biotech in the same space does raise significant questions as to the adequacy of Novo's pipeline.
At a smaller scale, Alkermes and Lundbeck have gone back-and-forth over Avadel, with bids rising from US$2.1bn to US$2.4bn and counting, to secure Avadel's Lumryz, a once-nightly oxybate drug for narcolepsy.
Despite holding no more than 20 positions at a time, three HB Biotechnology portfolio companies have been acquired since May 2025:
|
Company |
Asset(s) |
Acquirer |
Acquisition Price |
|
Small molecule TREM2 agonist for Alzheimer's Disease |
US$470m |
||
|
Ohtuvere for Chronic Obstructive Pulmonary Disease (COPD) |
US$10b |
||
|
Portfolio of Antibody-Oligonucleotide Conjugates (AOCs) |
US$12b |
Other recent notable acquisitions include:
- Merck – Cidara (US$9.2b): Gaining an antibody antiviral for influenza, a prophylactic alternative to vaccination.
- Sanofi – Blueprint (US$9.5b): Securing Akyavit for systemic mastocytosis and gastrointestinal stromal tumours.
- Genmab – Merus (US$8b): for their bispecific, Petosemtamab, for Head Neck Squamous Cell Carcinoma (HNSCC).
- Novo – Akero (US$5.2b) and Roche – 89Bio (US$3.5b): FGF-21 approaches for MASH –significant given the recent GLP-1-driven scepticism toward the field of MASH.
The message from acquirers is clear: innovation is scarce, capital is abundant, and pipeline gaps must be filled.
FDA Instability: A Residual Risk
The US FDA—long viewed as predictable and science-driven—has recently undergone unusually public disruption in the past 12 months:
- Robert F Kennedy Jr (RFK), a notable vaccine critic, was appointed secretary of HHS, sparking a sell-off in healthcare stocks.
- Dr Peter Marks, long-time head of CBER, was pushed out of the FDA.
- Dr Vinay Prasad (new head of CBER) resigned under pressure and was reinstated two weeks later; reports suggesting significant internal unrest.
- Dr George Tidmarsh (CDER) resigned amid controversy, alleging disagreements over accelerated-approval policy.
- Dr Richard Pazdur, a widely respected FDA veteran, has since been appointed to lead CDER, providing a much-needed stabilising presence.
This instability has translated into, what appears to be from the outside, sometimes inconsistent regulatory guidance, particularly in rare diseases:
UniQure (NASDAQ: QURE)
Initially aligned with the FDA on a Phase I/II dataset benchmarked to natural history controls for accelerated approval in Huntington’s Disease, the company was informed in October this would no longer be sufficient—despite RMAT, Breakthrough and other expedited designations and demonstrating a 75% slowing in disease progression over 3 years. For a fatal neurodegenerative disease with no disease-modifying therapies, the abrupt shift was striking, especially considering the Rare Disease Evidence Principles (RDEP) that were issued by the FDA in early September.
Replimune (NASDAQ: REPL)
Following the FDA declining to approve their drug, RP1 for advanced melanoma, citing trial design issues the FDA had earlier deemed acceptable, REPL has now resubmitted its BLA after additional analysis and the agency has accepted it with a (new) April 2026 PDUFA date.
For companies operating in small, hard-to-enrol diseases, these flip-flops complicate planning and add uncertainty. We expect stability to improve under the new leadership structure, but this remains a risk worth monitoring.
Outlook for 2026: Fundamentals in the Driver’s Seat
With tariff and pricing threats receding, a strong M&A backdrop, and genuine clinical breakthroughs emerging, we believe biotech enters 2026 with renewed momentum.
HB Biotechnology’s portfolio is well-positioned for the next phase. Several holdings approach major clinical and regulatory catalysts, and we continue to focus on opportunities where scientific fundamentals—not sentiment—drive long-term value creation.
Innovation cycles in biotech rarely unfold in straight lines, but the past six months reinforce a simple truth: when uncertainty clears, quality science and high-conviction stock selection win.

3 topics
12 stocks mentioned
