The freshest medtech opportunity on the ASX - CurveBeam AI
On Wednesday 23 August, Curvebeam AI (ASX: CVB) was listed on the ASX. The market for this kind of company remains hostile: biotech indices are close to their lows and the sector has missed the big tech/semiconductor rally which has supported indices this year.
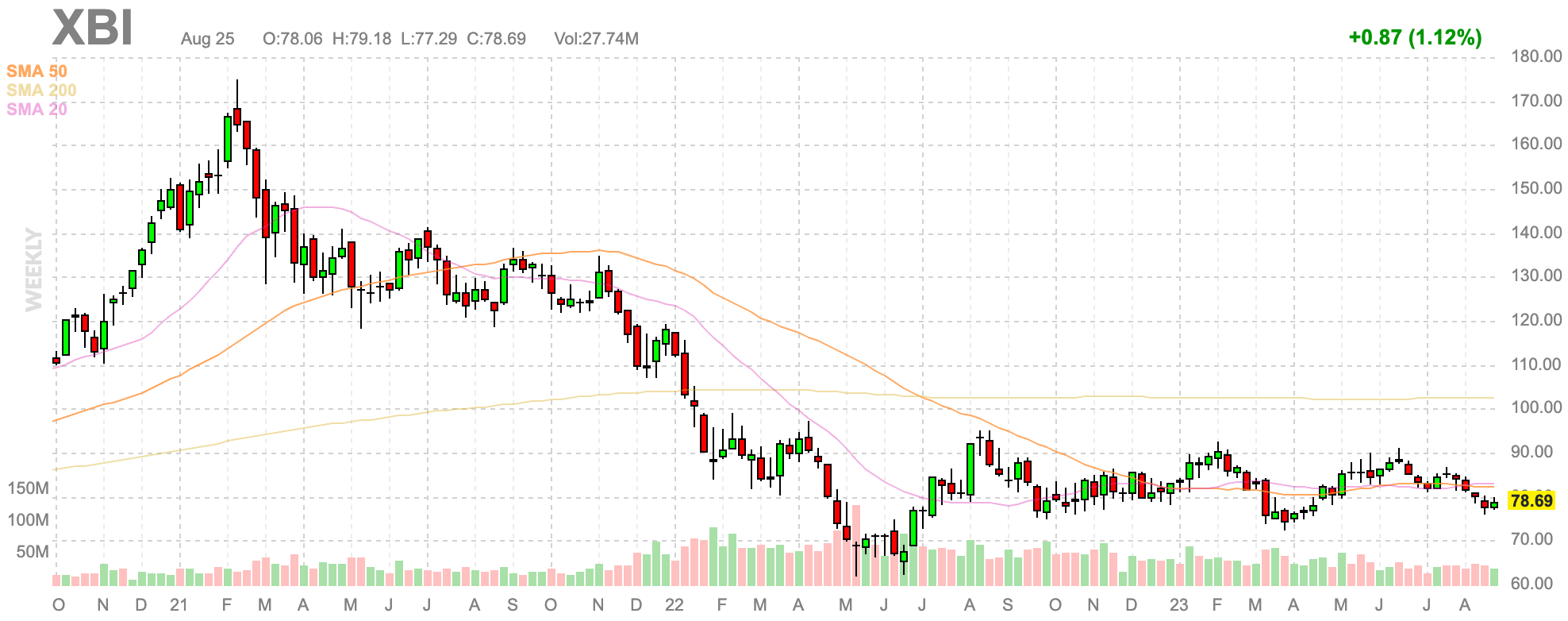
XBI, a US-listed biotech ETF
We expected this to be a volatile listing, and so it has proved.
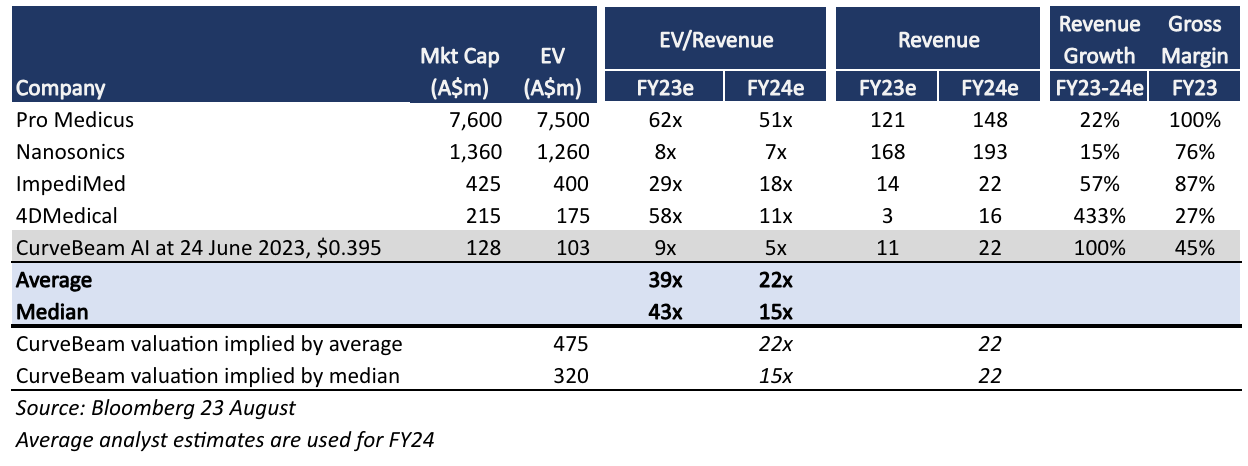
Curvebeam raised $25 million at a listing price of $0.48, representing a $155 million market capitalisation. We invested in our venture fund at $0.34 (last trade was $0.41). As a sense check on valuation, Impedimed (ASX: IPD) and Nanosonics (ASX: NAN), who also sell medical equipment and associated ancillaries to clinics, trade on ~18x EV/Sales, a 50% premium to CurveBeam's list price.
We have now invested over $12 million directly into early-stage life sciences businesses in Australia.
Our thesis rests on three parts
Firstly, a distribution relationship with Stryker should drive sales of CurveBeam's HiRise and InReach devices.
CurveBeam achieved revenues of $11.5 million over the year to 30 June 2023 (+55%), however this was largely achieved by senior management and a sales team of two. Over the next year, the firm is rolling out a distribution arrangement with Stryker's Foot and Ankle division, which would add hundreds of sales professionals and global coverage. We expect this to drive a step-change in growth, and encouragingly Stryker has already recorded their first sales.
Secondly, the company is rolling out software modules across its installed base which would increase the value of their devices for both orthopaedic practices (who can charge for additional scans) and CurveBeam itself.
And thirdly, CurveBeam is developing bone fragility and mineral density tests for which there is substantial unmet need in both screening and as part of pre-surgical planning.
Driving sales of Curvebeam's flagship product HiRise
CurveBeam’s HiRise scans a patient from the foot to the hip under gravitational load. It generates up to 66% less radiation than traditional CT units and is much smaller in size, allowing it to be easily placed in clinics, allowing surgeons to do scans in-house, generating additional revenue and making life significantly easier for patients.

Curvebeam's HiRise machine
CurveBeam made 18 HiRise device sales in FY23, bringing the total installed base to 45 machines since FDA approval two and a half years ago, a solid effort given this covered pandemic lockdowns and the cancellation of elective surgeries.
The market opportunity is immense. In the US there are roughly 6,000 orthopaedic practices, 6,000 imaging centres and over 5,500 hospitals that could make profitable use of a device like this.
Stryker relationship
Curvebeam's existing revenue was driven by a small in-house sales team and senior management, so we are excited to see what Stryker can achieve.
Stryker is one of the world’s leading medical technology companies with a market cap of $100 billion and revenues of over $19 billion, with Orthopaedics and Spine contributing around 55% of sales.
Last quarter, over 500 specialised sales reps from Stryker’s foot and ankle division were trained on the CurveBeam HiRise device and are now reaching out to foot and ankle surgeons across their client portfolios.
In the US there are 50,000 foot and ankle procedures done annually, and the partnership may expand to Stryker's hip and knee division, a market 40x the size of foot and ankle.
Stryker has a solid track record of incubating new technologies like CurveBeam, running a similar arrangement with Mako Surgical Corp before acquiring them in 2013 for US$1.6 billion.
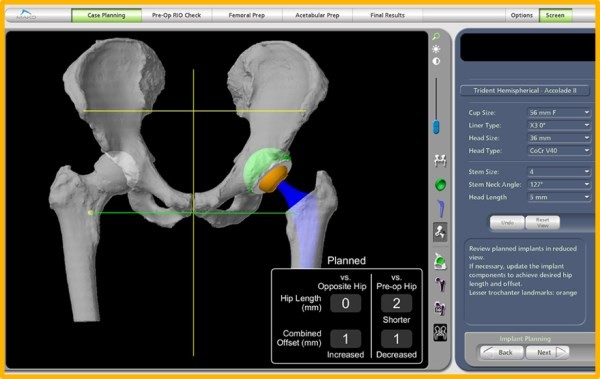
Mako developed a robotic-arm-assisted surgical system that is used for total knee, total hip, and partial knee replacements, incorporating 3D CT-based planning, where the surgeon uses a CT scan of the patient's joint to create a 3D model of the anatomy.
.png)
Mako 3D planning software based off a CT scan
Economics for clinics
The leaders of orthopaedic clinics will ultimately determine CurveBeam's success, and it is in these sales meetings that the future of the company will be decided. CurveBeam has to demonstrate significant value-add to justify the purchase of US$410,000 of equipment.
As part of the deal, CurveBeam can now leverage Stryker's balance sheet, offering financing for HiRise machines through multi-year commitments to purchase minimum quantities of Stryker products, removing the up-front capital hurdle.
Stryker used a similar financing product to sell the Mako system discussed above and was able to boost its installed base from 150 to over 1,800 units, and the Mako device costs twice as much as a HiRise, with no direct reimbursement incentives in place for surgeons.
Curvebeam can make use of existing reimbursement codes for the CT scan, Bone Mineral Density test for pre-surgical planning, and bone fragility assessments.
There are over 30,000 physicians in the United States specializing in orthopaedic surgery. These are busy clinics, with most seeing over 50 patients daily.
The average reimbursement for a lower extremity scan was US$140. At ten scans a day, the payback on a HiRise device is around one and a half years. If the device is financed over a 5-year term with Stryker, the purchase becomes accretive after only three scans a day, all while minimizing the times that clinicians need to refer patients to external imaging providers.

CurveBeam's equipment is designed to fit comfortably inside clinics
Bone Mineral Density and Fragility Testing
Osteoporosis occurs when bone mineral density and the resulting structure and strength of bone decrease significantly. It is common among people over 50, affecting 1 in 3 women and 1 in 5 men globally.
Approximately 2 million fragility fractures occur each year in the US, costing around US$19 billion annually.
Despite its prevalence, only about 20% of eligible women and 10% of eligible men undergo bone mineral density (BMD) screening, and a primary reason is the lack of access under Medicare reimbursement.
The most common technology used to perform BMD assessments today is an X‑ray DEXA scan, however, DEXA's accuracy in predicting fracture risk is questionable. While it measures bone mineral density, many elderly people who experience a bone fracture were not correctly diagnosed, even after a DEXA scan, which limits their treatment options under US Medicare and Medicaid. A better diagnostic process would expand the number of patients who can be pre-treated for bone fragility.
This is a clear area of medical need, as complications resulting from fractures are a major source of fatalities among the elderly. There is a desperate need for surgeons to better understand a patient's bone mineral density before planning surgical interventions.
Just 30% of people who suffer a bone break are diagnosed with osteoporosis using present-day BMD tests, the rest go undiagnosed. The current standard simply isn’t robust enough for comprehensive evaluation of community-wide fracture risk.
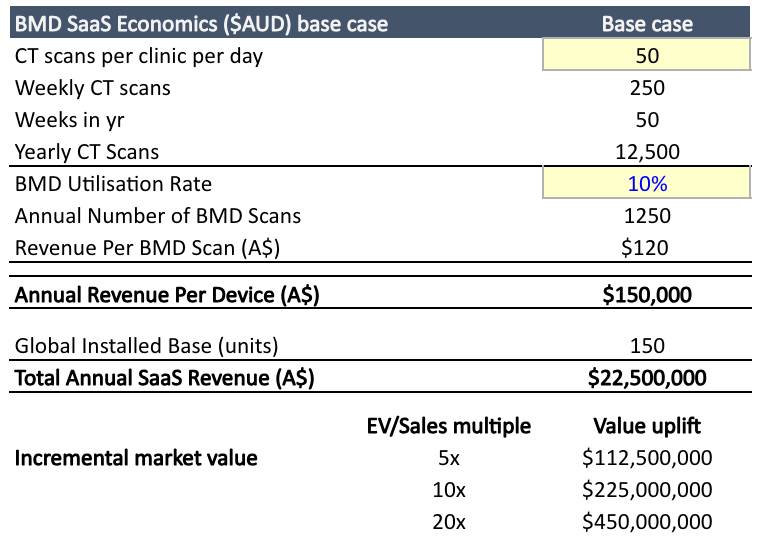
Back-of-envelope opportunity in bone mineral density. Note there is considerable downside risks to our base case. This assumes a significant expansion from ~45 installed units today, though we think this is achievable over time with Stryker distribution.
Bone fragility assessment with InReach

Both HiRise and InReach have the capability to measure bone mineral density and microstructure in patients with their OssView™ software module. An eight-year French study of a cohort of 2,000 women demonstrated that OssView can detect imminent and long-term fracture risks in individuals that today's standard testing often overlooks.
In 2022, the FDA granted CurveBeam Breakthrough Designation for InReach, with FDA clearance targeted for FY24.
Unlike the DEXA scan, CurveBeam’s InReach test doesn’t require a trained operator. The patient inserts their wrist into the device and in 1-2 minutes a 3D hi-resolution scan of the wrist bone is complete. A report is then sent to the physician via the cloud using Curvebeam's AI software to detail the patient's potential for fractures.
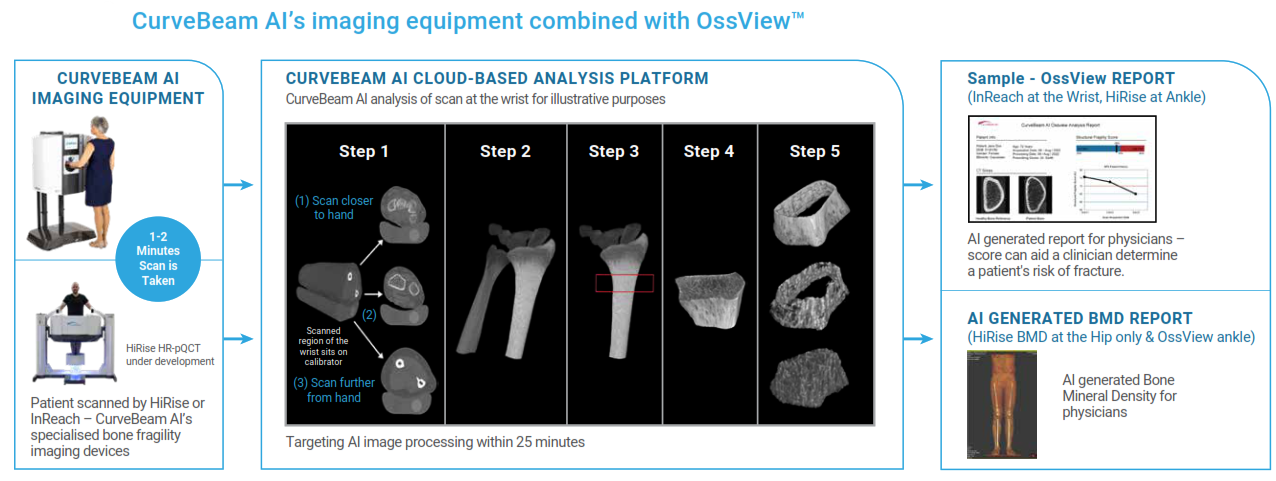.png)
Source: CurveBeam Prospectus
In FY24 CurveBeam plans to submit an application to the FDA for approval to run the same test on the HiRise, aiming for clearance by FY25, increasing the value of their installed base of HiRise machines for both clinicians and the company.
This adds a significant revenue opportunity for CurveBeam, with the fragility screening market worth >A$2.5 billion, and much of this would be recurring every 24 months.
Building a portfolio of high-margin software modules utilizing Curvebeam's installed base
CurveBeam is growing its portfolio of high-margin, recurring software streams from the underlying HiRise installed base in the market.
.png)
CurveBeam’s regulatory pipeline for software modules. Source: CurveBeam Prospectus
CurveBeam is developing a bone mineral density assessment tool generated by HiRise CT scans specifically to assist in presurgical planning.
Revision surgery caused by failed implants from undiagnosed bone fragility is a major issue, costing time and money to readmit a patient and redo the implant, not to mention increasing the risk of complications.
When bone fragility is identified early in pre-planning, surgeons can reinforce or cement the bone to reduce the risk of implant failure.
Currently, surgeons avoid sending patients off for bone mineral density tests as part of presurgical planning as they receive no revenue on scans done outside the clinic. These tests can also take weeks to get results, creating an unwelcome bottleneck.
With CurveBeam's device, surgeons can bill for both the CT scan and the bone density assessment in one go, opening up new revenue streams and reducing expenses linked to follow-up surgeries due to initial procedure complications.
The potential market size for BMD testing in the US is around A$2.7 billion. CurveBeam is targeting FDA filing for BMD in FY24 with clearance in FY25.
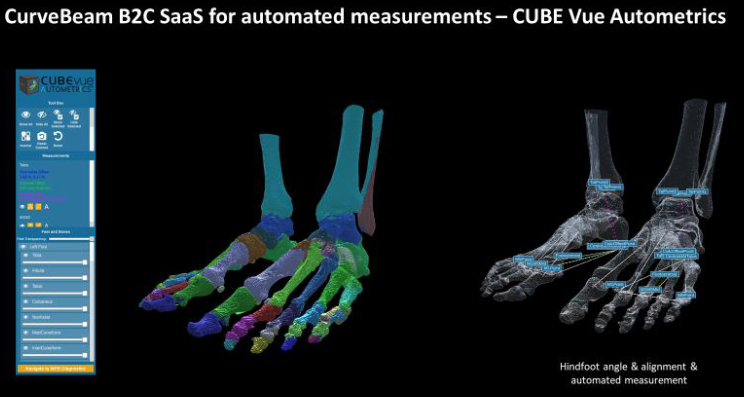.png)
CubeVue software on HiRise for bone segmentation analysis
Curvebeam is also working on 'CubeView', a bone segmentation analysis tool for foot and ankle surgeons.
A CT scan of the foot reveals over 25 bones and 33 joints. Manually segmenting these bones and accurately gauging bone geometry and alignment can take a skilled practitioner 6-8 hours. The process is so onerous that most surgeons opt for simpler, less detailed analyses. CubeVue crunches 16 hours of anatomical measurements and the pre-surgical planning work in under 20 minutes.
Summary
The inevitable volatility involved in listing at such a difficult time is balanced by the fact this is listing very cheaply, particularly when compared to local medtech comparables.
CurveBeam has made a lot of progress. The company has:
- developed a unique, high-tech, hardware platform to solve a serious problem in orthopaedics,
- signed an agreement with Stryker which will increase their sales coverage by two orders of magnitude, and offers important validation of their technology from the industry giant (who in the past, has also been acquisitive)
- developed value-adding software modules to satisfy other areas of unmet need, increasing the value of their hardware to both themselves and their customers.
The management team has form. Chief Technology Officer Arun Singh led the development and commercialisation of the I-CAT system, a 3D conebeam CT imaging platform allowing dentists to rapidly scan the teeth, soft-tissue, nerve and bone structure of patients in their own clinics, with over 9000 systems installed globally. There are clear parallels to CurveBeam's approach to orthopaedics.
Our strategy is simple: stay invested while the firm expands its distribution through Stryker, rolls out high-margin software modules, and develops their bone mineral density and fragility screening tests in both the elderly and as part of pre-surgical planning.
These are ambitious, but achievable plans. If the market values CurveBeam anywhere near its slower-growing peers on the ASX, there would be a substantial value uplift over the coming years.
2 topics
3 stocks mentioned

