The new medical technology redefining treatment in cardiac health
We attended the Atrial Fibrillation Symposium in Boston this past January, and it was great to see the buzz around Pulsed Field Ablation (PFA) technology in person. While it has been somewhat evident from progress in European hospitals that PFA is set to become the gold standard of care in the treatment of atrial fibrillation, it is now our view that PFA will undoubtedly become one of the most widely used energy approaches for the treatment of irregular heartbeats globally. As PFA has only just now become commercially available in the U.S. (after being available in Europe for the past few years) we are still in the early stages of a long transition towards this treatment.
What is atrial fibrillation?
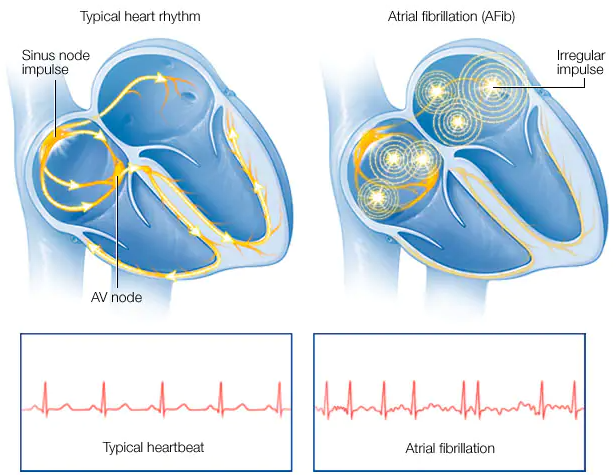
Atrial fibrillation (AF) is an irregular and often
rapid heartbeat that disrupts the heart's ability to pump blood efficiently.
It's a significant health concern with a rapidly growing prevalence, especially among
ageing populations. An irregular
heartbeat often leads to increased risk of cardiovascular disease such as blood clots and stroke.
What is the current state of the market?
Treatment of AF is the biggest part of the $8 billion electrophysiology market, making up over $5 billion in procedures, and growing as patient volumes continue to rapidly expand. Over the past decade, minimally invasive catheter-based treatments for AF have been growing in the low double digits, which is widely anticipated to continue at least through the end of the decade. This has been driven by i) increasing prevalence of AF, ii) more data supporting early rhythm control, and iii) recent guidelines changes supporting catheter ablation as first-line therapy in select patients.
Conventional ablation systems use thermal energy (i.e., temperature) to block abnormal electrical signals which are causing the irregularity in a patient's heartbeat. There are currently two types of ablation systems:
- Radiofrequency ablation, which uses heat produced by high-frequency radio waves, and
- Cryoablation, which uses freezes nerves with liquid nitrous oxide or argon gas
The biggest risk of these current systems is that they can cause indiscriminate tissue injury in surrounding areas of the heart, which can result in complications in adjacent structures like the esophagus and phrenic nerve. These systems also require a significant amount of time to ablate large regions of heart tissue, with some interventions taking 5 hours or more.
What is Pulsed Field Ablation?
PFA, on the other hand, is fundamentally different. It uses electrical energy (a non-thermal energy source) to cause what is known as 'electroporation' - which simply means opening holes in the cell membrane - and leads to cell death, blocking the electrical signals causing the irregular heart beat. Because cardiac muscle cells have a lower threshold to irreversible electroporation, this technique can be used to preferentially target cardiac cells while adjacent cells remain unaffected.
PFA is a more selective, faster and mechanistically safer technology compared to the current thermal energy methods.
In all medical technology advancements, safety is the primary concern. In this regard, PFA has so far been a homerun. But it is the clinical data above and beyond the impressive safety profile that has got us really excited about PFA's potential to grow the market. PFA enables faster procedures, more homogenous outcomes across operators, and same-day patient discharge which dramatically lowers the cost to the hospital and insurers. All of this combined is likely to drive greater cath lab capacity, increased referrals from cardiologists, and more patients willing to undergo the procedure. With a broader patient funnell more physicians will adopt the technology, and as one high volume physician told us at the AF Symposium, "once docs use PFA, they'll understand how game-changing it is, particularly its ability to make cardiac ablations safer, easier, quicker, and reproducible". This particular physician, while only an 'n of 1', now uses PFA in >90% of his AF cases.
Who are the key players?
The current market leaders in PFA technology are Boston Scientific (NYSE: BSX) with their Farapulse catheter and Medtronic (NYSE: MDT) with their Affera system. Farapulse was first to market receiving the European CE Mark in January 2021, and each of these systems have since been approved in both the EU and more recently the United States. Because of the size of the reward on offer, the market will continue to get more competitive. Deep pocketed players such as Johnson & Johnson (NYSE: JNJ), who recently got CE Mark in Europe for their PFA catheter called Varipulse, and Abbott (NYSE: ABT) are chasing down the incumbents in the space.
This will certainly not be a winner take all market, but we do expect the cost of market entry (upfront R&D, clinical trialling, and regulatory burden) to become too steep for multiple new players in the short term. While each of the PFA catheters have their own pros and cons, one key aspect that we have determined to be critical in the ultimate market share battle is the mapping system attached to each PFA technology. Abbott’s EnSite, Johnson & Johnson’s CARTO3, and Boston Scientific’s Rhythmia are the primary mapping systems used today, though multiple players are all developing new mapping systems. For now Boston Scientific are using an 'open mapping' approach, allowing their catheter to be used with any of the competitor's mapping systems, although we expect that to change as Abbott and Johnson & Johnson come to market in the next few years.
Conclusion
Ultimately, our view is that the PFA market will rapidly become the standard of care in the $5+ billion market for the treat of atrial fibrillation. Our due dilligence checks with doctors at the Atrial Fibrillation Symposium in January this year only added to our belief in the technology, which has been proven out in clinical data over the past half decade. There is room for multiple players here, but Boston Scientific's technological advantage and first-to-market head start provides it with the best shot at getting comfortable in physician's hands.
Want to learn more?
Follow Cordis on LinkedIn or here on LiveWire for thoughts, updates and ideas in medical technology. Or feel free to reach out to me directly, I'm always happy to catch up and chat!
4 topics
4 stocks mentioned

