Wilsons top six healthcare picks for 2022
As a holiday send-off, here is a list of the top six stocks in Wilsons healthcare coverage that excite us about coming back from the break. Those stocks are: Immutep, Aroa Biosurgery, CSL, ImpediMed, Telix Pharmaceuticals and Silk Laser Clinics. In every case, we see 2022 as the year when the proof of a pivotal concept, clinical asset or business model unlocks a material component of valuation. Alongside this publication we note material price target upgrades for CSL, Telix and Silk Laser. ImpediMed’s inclusion follows the pre-publication of its pivotal PREVENT trial which proved that subclinical lymphoedema after breast cancer therapy is avoidable.
Immutep (IMM) - Price Target: $0.91 per share
Immutep benefits from external validation of LAG-3 target in Immuno-oncology.
LAG-3 validated as the third important Immuno-oncology target. Immuno-oncology has dramatically changed how we treat cancer and the survival outlook for patients with late stage and metastatic disease. Since the first approval of an immune checkpoint inhibitor (Bristol Myers Squibb’s ipilimumab) in 2011 we have seen only two target pathways arise in this field; CTLA-4 (ipilimumab’s target) and PD-1/PD-L1 (the target/s of six approved checkpoint inhibitors). To date, this class of drugs (immune checkpoint inhibitors) annualises >US$30B in sales from seven approved assets. Merck’s pembrolizumab (anti-PD-1) first approved in 2015 is a blockbuster that is now used in >13 cancer indications, annualising US$16B in sales (Figure 1). LAG-3 is the most advanced novel immune checkpoint target, which could see its first targeted drug approval in 1Q’22 via BMS’ relatlimab (anti-LAG-3 drug), adding LAG-3 to CTLA-4 and PD-1/PD-L1 as the third important immune checkpoint target pathway in oncology.
Figure 1. Global sales of key approved immune checkpoint inhibitors
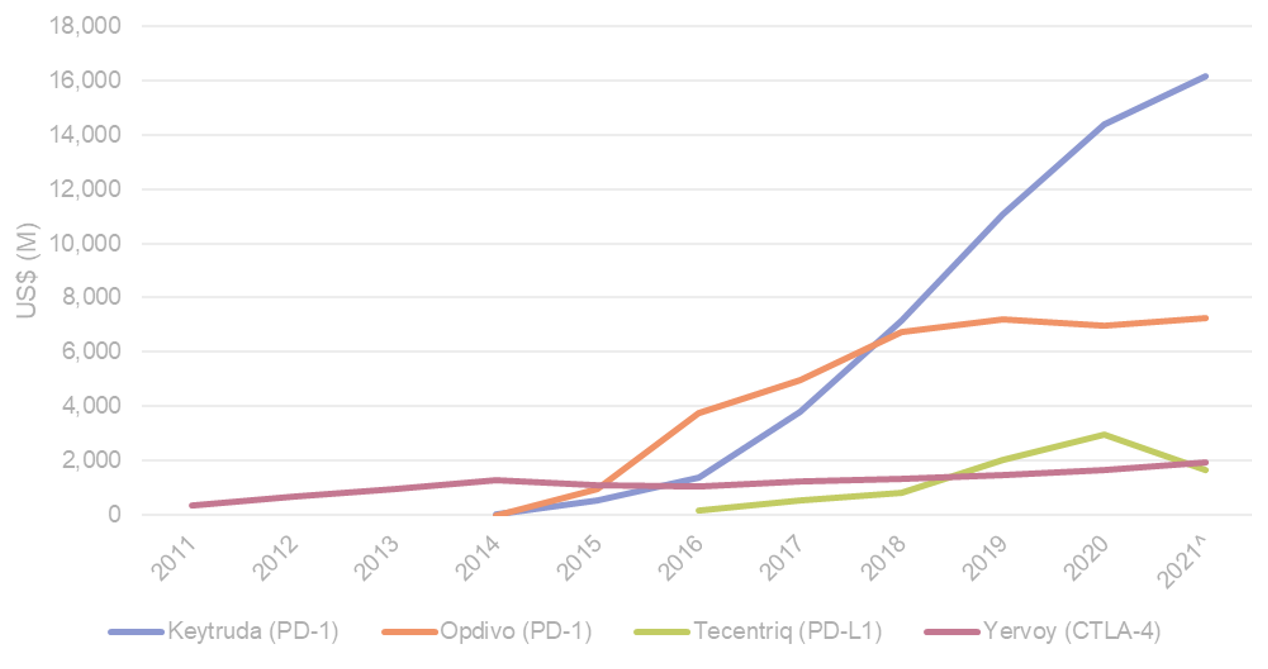
^2021 figures based on annualised 1H21 reported sales. Source: Wilsons, MSD, BMS, Roche.
Relatlimab approval is a re-rating catalyst for Immutep. Regulatory approval of BMS’ anti-LAG-3 asset validates Immutep’s core pipeline target, LAG-3. Approval of relatlimab by FDA we believe triggers a hive of activity and interest in LAG-3 directed assets within the pharmaceutical sector. Relatlimab’s PDUFA date with FDA is 19 March 2022. Immutep’s lead asset, Efti, does not modulate LAG-3 in the same manner as relatlimab. The asset IMM have outlicensed to Novartis (LAG525) does act in this manner however and therefore relatlimab approval could be seen as a positive readthrough for this asset. Efti, on the other hand, is mechanistically unique and can ‘boost’ immune response even in patients that lack LAG-3 expression on target T-cells, which expands Efti’s applicability and potential to synergistically enhance other IO regimens such as Keytruda. The ability to expand the market of an established blockbuster, as opposed to compete against it, is a key part of our positive investment thesis on Immutep.
1H’22 an important readout period for IMM’s non-small cell lung cancer (NSCLC) program; potential licensing catalyst. The Phase II TACTI-002 study (in partnership with MSD) has now completed extension of Cohort A (1st line NSCLC) to 110 patients (previously 36). We expect readouts from these additional 74 patients in 1H’22 with respect to progression-free survival (PFS) and response rate (ORR) – key efficacy measures dictating Phase IIb/III trial progression. If IMM can replicate initial data (n=36) in the expanded cohort we anticipate this would be a catalyst for licensing activity for Efti in this indication, noting that the next phase of clinical development requires significant investment best suited to strategic pharma partnership.
New clinical trial programs get underway: AIPAC-III in breast cancer. In CY22 we also see Immutep deepen their clinical development pipeline with their first Phase III registrational trial in metastatic HR+/HER2- breast cancer get underway (IND approval expected early 1H, recruitment underway before 2H’22). This trial, coined AIPAC-III, will evalute the potential for Efti to add to existing standard of care chemotherapy to enhance response rates and survival (without added toxicity), which we have seen to date from the Phase IIb AIPAC data, specifically in younger (<65yr) patients. We model a potential opportunity for Efti in those <65 years of US$750M peak sales in this indication, where very little innovation is occurring.
TACTI-003 in HNSCC. We expect 1H’22 to be a key recruitment period for IMM’s Phase IIb study in 1st line head and neck cancer (HNSCC) in partnership with Merck (MSD). TACTI-003 aims to extend on the positive data in 2nd line patients from TACTI-002, and has the potential to support an accelerated path to market should data be outstanding (i.e. consistent with TACTI-002 outcomes) further buoyed by their FDA Fast Track designation status.
Valuation. Our risked PT of $0.91 per share is a SOTP real options valution comprised of a) Efti in breast cancer ($0.30/sh); b) Efti in HNSCC ($0.09/sh) and c) Efti licensing in NSCLC ($0.53/sh). No other assets included. Unrisked PT is $2.33 per share.
Aroa Biosurgery (ARX) - Price Target: $1.75 per share
Surgical reopening, SYMPHONY launch and MYRIAD traction drives growth for Aroa
MYRIAD salesforce formed; CY22 key commercial ramp year. Aroa now have a MYRIAD-focused US direct sales force of >20 reps to drive MYRIAD adoption and have the product/s on formulary with several key GPOs. The growth expected from the MYRIAD franchise is supported by the newly added Morcells™ product approved in April 2021, to complement the MYRIAD Matrix sheet product. We have already seen excellent traction with Morcells and expect it to continue to contribute positively to ARX’s gross margin given it is a product manufactured from the ‘scrap’ or waste matrix sheeting (>95% GM). As a reminder, >50% of ACell’s business (prior to Integra acquisition) was from their particle product (MicroMatrix® akin to Aroa’s Morcells™) (see Figure 2). A dearth of these types of products in the inpatient wound market gives Aroa an excellent platform for sales traction and adoption.
Figure 2. MicroMatrix accounts for ~50% total ACell revenues
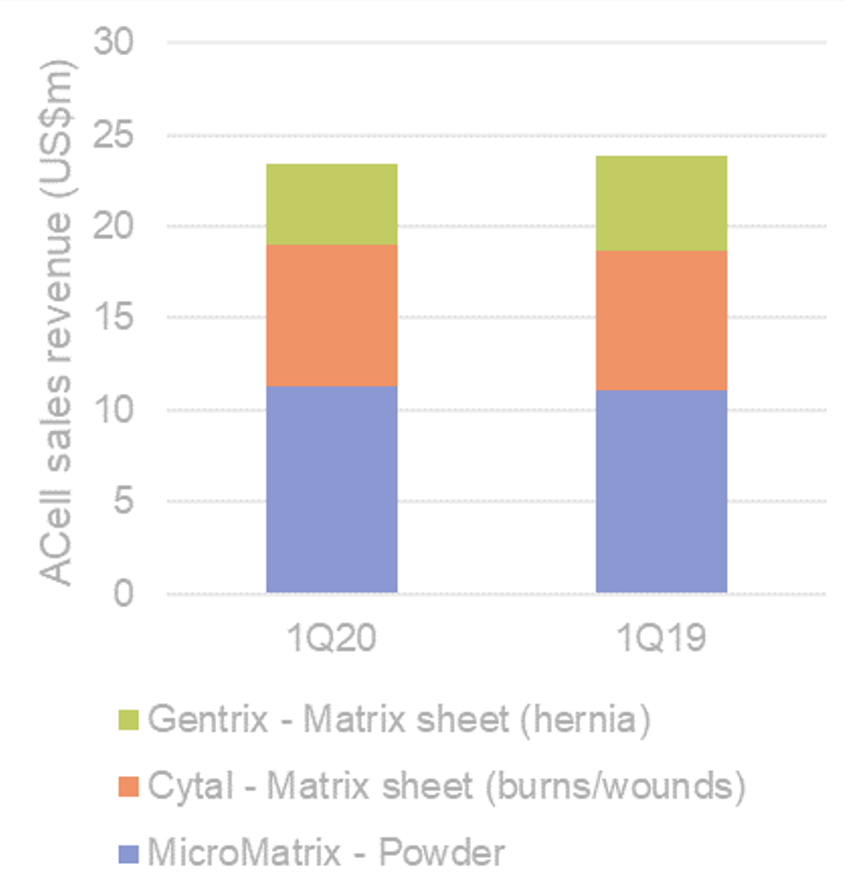
Source: ACell, Wilsons.
SYMPHONY launch in 1Q’22 expands into new market for Aroa. The launch of Aroa’s new skin substitute product, SYMPHONY, which received FDA clearance in July 2020, is due in 1Q’22. The delay was initially to accommodate potential changes to CMS reimbursement in outpatient wound care which did not eventuate, which may have guided Aroa in pricing determination. Nevertheless, Aroa can proceed with the knowledge that SYMPHONY is a product that can/will be used in preference to biologics such as Epifix®, AlloDerm® and Integra® due to pricing benefits. We anticipate Aroa can offer SYMPHONY at a ~40-50% discount to existing biologic skin substitutes which typically retail at US$6,000-US$10,000 per device. Aroa estimates a SYMPHONY TAM of US$1B with a focus on hard to heal wounds in patients with comorbidities (i.e. diabetic foot ulcers, venous leg ulcers). This is an area of wound care responsible for the most CMS spending, with diabetic foot ulcers alone estimated to cost CMS >US$6.2B/yr to manage. There is potential for SYMPHONY to expand the adoption of Endoform product use also given product complementarity. We model material SYMPHONY contribution from FY24.
TELA traction with GPOs likely to continue and expand. We view TELA Bio’s ability to snag additional GPO accounts (i.e. Vizient, Ascension) as likely given the traction they have seen with their existing GPO wins (HealthTrust, Premier) and the tenacity of their sales team/management. As a reminder, TELA continue to average 137% of quarterly revenues per HealthTrust GPO account versus others (Figure 3) which makes for an effective use of sales & marketing resources.
Figure 3. TELA Bio account revenue split estimates between HealthTrust GPO (HPD) and other/IDN accounts
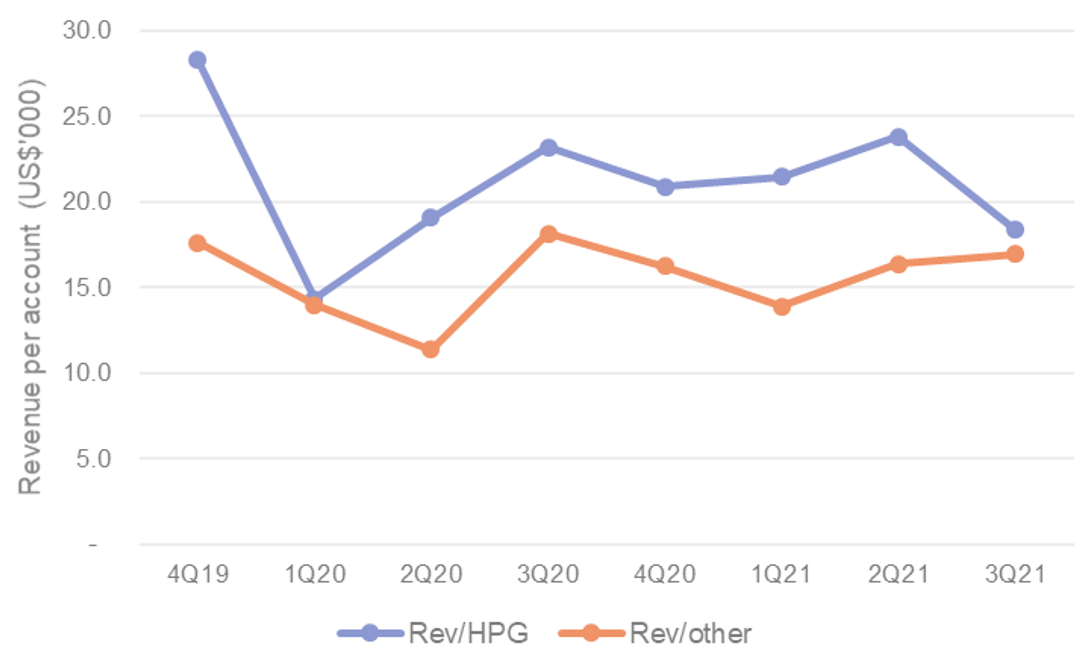
Source: Wilsons estimates, TELA Bio.
OviTex portfolio continues to ride robotic trend in hernia. The Ovitex LPR product range is uniquely positioned in the hernia market in that it is a biological material that has the strength to be used in laproscopic and robotically-assisted surgical repairs. Other biologics in this category do not have the strength or flexibility to be used in this setting to this degree. We expect incremental launches in the OviTex LPR range to increase robotics share in hernia.
R&D pipeline well funded and has found a home. Aroa’s dead space management product currently under development (~CY23 launch expected) is now well capitalised to ensure the clinical studies can be conducted swiftly and in full. Further, TELA Bio have committed to taking ownership of the new system, pending FDA approval, to add it to their existing OviTex product portfolio. We have not done extensive market evaluation of this product but understand there is a sizable opportunity in the area of surgical complication management (e.g. surgical wound dehiscence in plastics, orthopaedics, obstetrics).
Valuation. Our price target of $1.75 per share implies a ~13.2x FY23e EV/Revenue. Aroa currently trades at 7.5x FY23e EV/Revenue which is a material discount to ASX wound care peer PolyNovo (PNV) at 15.8x on the same metric.
CSL Limited (CSL) - Price Target: $350 per share
Re-establish dominance in plasma during the biggest year for R&D in the company’s history
Structurally stronger in plasma post-COVID driven by US channel presence and dominance in subcutaneous IG (HIZENTRA). Plasma collection dynamics should continue to normalise over the next 12-18 months. Our view is that higher collection costs are likely to endure as donor fee expectations could remain elevated and collection networks must expand into new territories. Our sense is that CSL Behring has strengthened competitively in the US market and that could persist. Structurally we expect the IG mix can trend further towards subcutaneous. In that case CSL market share should benefit as HIZENTRA is preferred to other 20% IG options (CUVITRU, XEMBIFY).
Major market filings and approvals for EtranaDez (haemophilia B gene therapy). All patients enrolled in HOPE-B were expected to be through 78-week follow-up by the end of Sep-Q setting up BLA/MAA filings in 1Q 2022. EtranaDez has Orphan Drug designation and is eligible for biologic exclusivities if approved in late 2023. We have EtranaDez sales in our model from FY24e expecting the asset to do best in the under-served, late-adolescent / early-adult cohorts. In that sense we see EtranaDez as accretive to CSL’s haemophilia franchise and a conduit to IDELVION therapy. EtranaDez is also materially accretive to Behring’s gross margin if pricing expectations (US$2M/dose) are realised.
Pivotal data from CSL’s AEGIS-II Phase III trial. Phase III trial enrolment has passed 80%. We expect a third interim analysis (efficacy driven) to report around Aug-22 with top line results potentially by end-2022. Note the primary endpoint in thi9s study is short: a 90-day follow-up recording time to first occurrence of any component of MACE (cardiovascular death, myocardial infarction or stroke). In recent months the mechanistic basis for CSL112 (plasma derived apolipoprotein A1) has firmed as more data is published from Phase IIB (AEGIS-I). Our CSL forecasts do not include CSL112, the financial outlook for which we assess in detail, early 2022.
Table 2. CSL Behring earnings summary (FY20-FY24e)
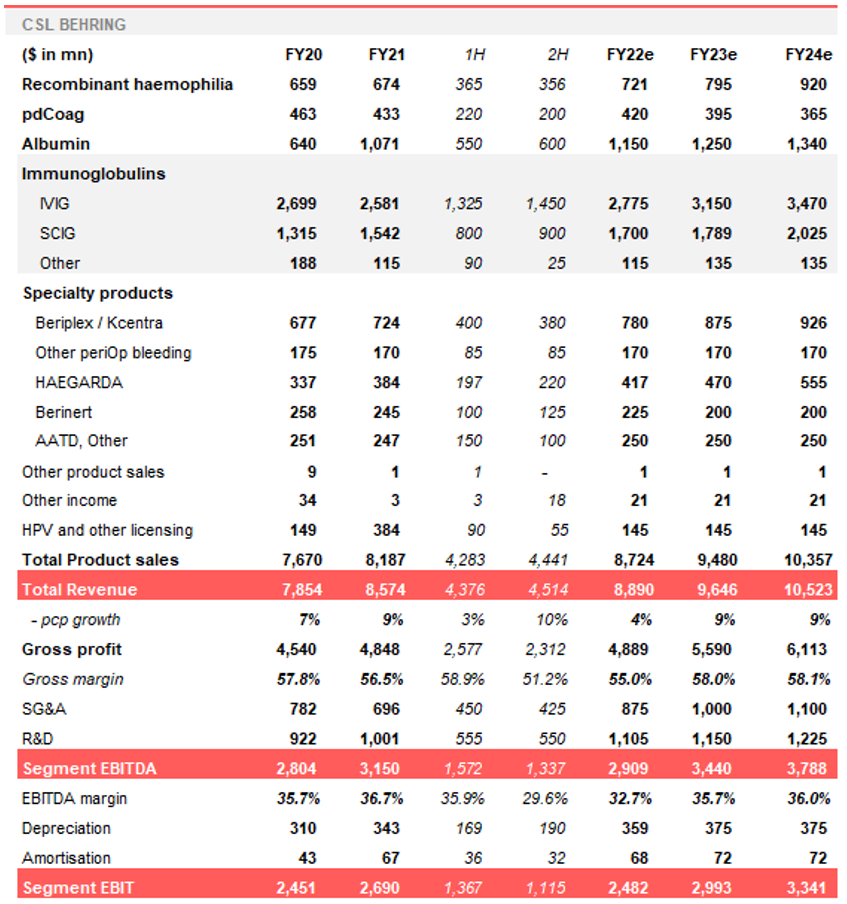
Source: Wilsons
The anti-FcRn movement in CIDP comes to nothing. The prospect for ‘anti-FcRn’ fear-mongering is reasonably low over the next 12 months with ArgenX’s Phase III ADHERE trial in CIDP delayed into mid-2023. Our view on that trial is that the autoantibody component of CIDP pathogenesis is too weak to expect much from efgartigimod. IG therapy should remain standard of care in CIDP given its ability to address immune-driven inflammation. We are expecting ArgenX’s efgartigimod to be approved by FDA on the 17th December for myasthenia gravis (MG). Expert opinion remains divided as to whether FDA’s approval should be conditional on a risk evaluation and mitigation strategy (REMS). A REMS would impose a hard barrier to off-label use in CIDP or other adjacent indications.
Garadacimab asset building towards Phase III read in 2022. CSL’s first internally developed, fully humanised monoclonal antibody candidate captured attention when it debuted with stunning Phase II data at the 2019 R&D day. Its Phase III in hereditary angioedema (HAE) has recruited ahead of schedule and reports top-line in Jul-22. HAEGARDA resilience in the face of Takeda’s TAKHZYRO has surprised. If approved, garadacimab brings a new mechanism of action to HAE prophylaxis (targeting FXIIa which acts earlier in HAE attacks that TAKHZYRO’s target, kallikrein).
Valuation. New price target of $350 per share equates to 30x FY23e EBITDA (WILSe: US$4Bn). Note that approximately 10% of our valuation stems from three ‘risked’ R&D programs: CSL112, EtranaDez and garadacimab.
ImpediMed (IPD) - Price Target: $0.31 per share
NCCN guideline inclusion launches reimbursement and extensive adoption of SOZO
Top line results revealed in PREVENT trial. The randomised, controlled trial enrolled 1,200 breast cancer survivors and randomised 963 of them to prospective surveillance programs using bioimpedance (BIS) (with ImpediMed’s SOZO + L-Dex® technology) versus tape measure (TM) with the intention of early detection and prevention of lymphoedema, a key complication follow breast cancer surgery. At 3 years’ follow-up BIS proved to be the superior technology, associated with a 59% relative reduction and an 11% absolute reduction in the rate of lymphoedema progression. Importantly, the benefit of using BIS was preserved after adjusting for a range of pre-disposing risk factors. For the primary endpoint, BIS was associated with a 7.9% rate of progression to lymphoedema compared to 19.2% progression for TM. This difference was statistically significant (p = 0.016) (Figure 4).
Figure 4. BIS (using L-Dex®) significantly reduced the proportion of patients progressing to chronic lymphoedema vs. Tape Measure (TM) in PREVENT trial
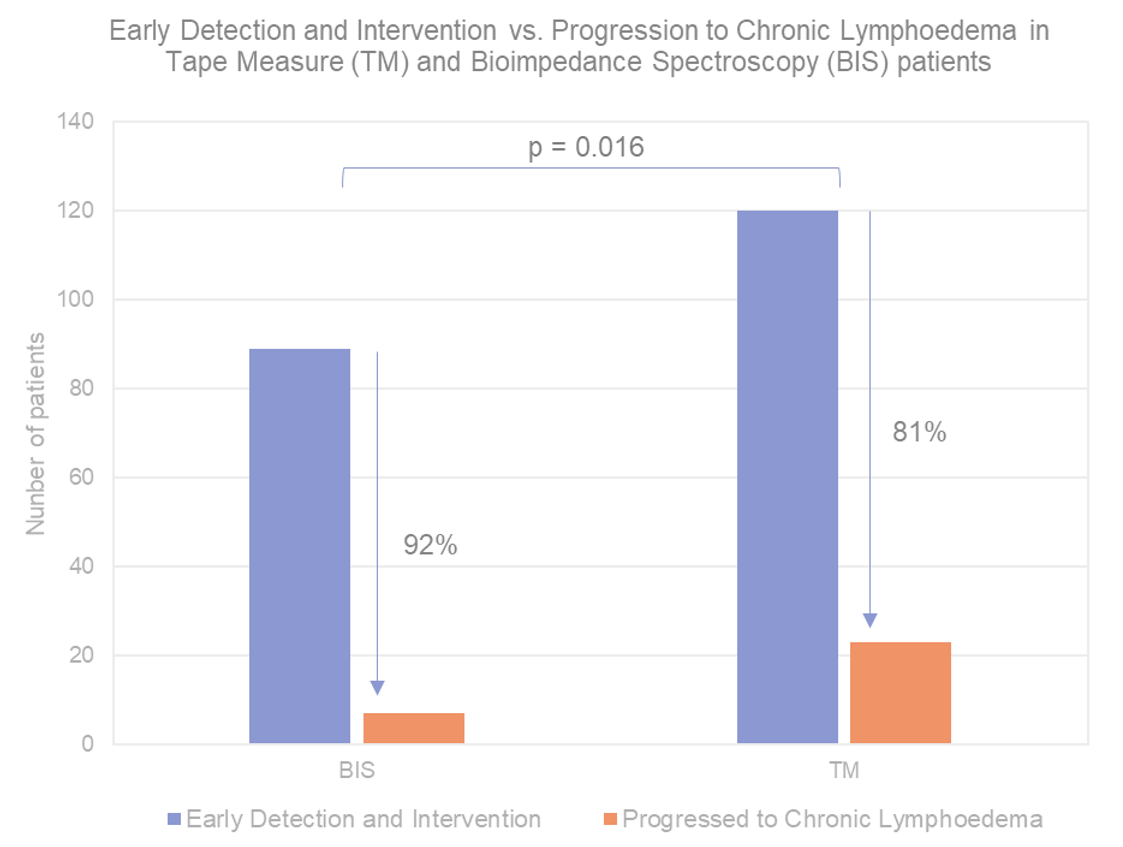
Source: Ridner et al. (2021) preprint, Wilsons.
NCCN guidelines key catalyst for SOZO. Following peer review of the PREVENT trial, level 1 evidence may become available. This supplies ImpediMed with further valid reasoning to request changes to lymphoedema NCCN guidelines to specify use of BIS in lymphoedema early detection and management. We have recently seen changes to NCCN guidelines regarding inclusions for PSMA-directed prostate cancer imaging, representing a key catalyst for Telix’s Illucix® product, particularly in driving private payer reimbursement. With lack of competitors in the multiple spectroscopy space (as opposed to singular), inclusion of the BIS technology in NCCN guidelines presents as a sizeable opportunity for SOZO.
Sound revenue model with potential to move to volume-based pricing. Current revenue model for SOZO sits at US$5K for each device with US$12-15K per annum per SOZO as an annuity (90% gross margin). Potential changes to NCCN guidelines alongside evidence from the published PREVENT trial have potential to shift the current model to a volume-based pricing approach. The PREVENT trial showed benefits across all risk categories suggesting that all breast cancer survivors should be offered prospective surveillance for subclinical lymphoedema and that it should be instituted as early as possible, post-treatment. Widespread payer support should encourage broad utility across breast cancer-related lymphoedema and emerging use in other FDA-cleared indications (bilateral disease, lower limb). This provides IPD with genuine latitude to move further towards a volume-based approach over time.
Healthy cash position. Following completion of a recent $35M capital raise, ImpediMed head into 2022 with over $50M in cash. This allows for continued sales in the lymphoedema sector, through to break even in FY24e. Additionally, it includes the capacity for IPD to continue to focus on the development of SOZO into heart and renal failure indications.
Cracking the renal failure market. SOZO was granted Breakthrough Device Designation by the FDA for a proposed indication in renal failure. Currently there is no scientific method of measuring fluid volume, which forms the basis of a patient’s dialysis treatment (i.e. how much fluid is removed), nor a method to easily keep track of nutritional deficiencies (such as albumin). Both factors contribute to high mortality rates within the first 3 months of initiating dialysis treatment. Given kidney dialysis centres have a large fixed cost component, the loss of patients to death and hospitalisation is problematic for network profitability. With the ability to accurately measure fluid with SOZO, the risk of mortality due to hypovolemia, hypervolemia and nutritional deficiencies is reduced. The top two dialysis providers – DaVita and Fresenius operate over 5,000 centres across the US, providing over 65M treatments annually.
Valuation. Price target maintained at $0.31/share having recently de-risked the PREVENT trial outcome in our model. Our IPD PT is a risk-weighted DCF that maps to the US lymphoedema and heart failure businesses only. Un-risked valuation is $0.65/share (implies ~$1B in EV) and excludes renal for the time being.
Telix Pharmaceuticals (TLX) - Price Target: $10.35 per share
The commercial organisation lets the numbers do the talking
ILLUCCIX launch dynamics in 2022 can re-rate the stock beyond DCF. Telix recently updated its ILLUCCIX USA TAM estimate to US$725M (from US$575M) and has aspirations to capture 40% of that within three years from commercial launch. Our peak sales forecast of US$200M may be conservative but makes allowance for modest ASP decline and competitor launches including Lantheus, Novartis and Blue Earth (Bracco). We remain comfortable with our FY22e forecast of A$77M which is effectively a 9-month contribution with full market access expected from April. At $6.60 per share Telix trades on 24x FY22e but just 12x FY23e (EV/revenue, with revenue doubling to A$146M in FY23e). Our sense is that the valuation can remain north of 20x EV/revenue on the basis of ILLUCCIX sales execution and that implies share price growth towards $10.35 per share (noting our un-risked TLX valuation is $14/share).
TLX591 parked safely within the ProstACT Phase III campaign. The start of the ProstACT trial series is a positive for valuation in that it will settle design controversies and sideline the efficacy versus toxicity debate for the duration of the pivotal 591 study. As a reminder, ProstACT will test standard of care (SOC) ± a fractionated dose of TLX591 (2 injections of 45 mCi/m2 each, 14 days apart). The most recent trial update confirms that the SOC definition includes at least one prior line of taxane chemotherapy. There is no cross-over in the trial so the availability of an active SOC should help preserve the primary endpoint. The selected dose was associated with median survival of 42.3 months (n = 17) in Phase I/II. As a comparator, Novartis reported 15.3 months for 177Lu-PSMA-617 in the VISION Phase III. At 2x45 mCi/m2 TLX591 53% of patients required platelet transfusion to manage haematologic toxicities. Expert opinion suggests TLX591 would need a ≥50% efficacy advantage over 177Lu-PSMA-617 to win favour with prescribing medical oncologists.
TLX592 takes the lead in the prostate cancer therapy narrative. TLX592 combines an optimised monoclonal antibody targeting moiety with an alpha-emitter (225Ac). It extends the reach of PSMA-directed radiotherapy in both directions, relative to TLX591 whose beta-omitting activity is best suited to the metastatic patient failing AR pathway inhibitors, taxane chemotherapy or both. In the earlier stage patient we see neoadjuvant opportunities – using TLX592 in patients undergoing radical prostatectomy or pelvic lymph node dissection. In the later stage patient progressing after TLX591 and/or 177Lu-PSMA-617, TLX592 may serve as a targeted salvage therapy, which (unlike Bayer’s XOFIGO) may address both bone-resident and visceral metastases.
ZIRCON Phase III – synergies in urology with optionality on the CAIX target. TLX250-CDx has FDA Breakthrough Device Designation because it looks likely to become the first and only non-invasive modality for assessing renal cancer biology. If approved the product will obviate tens of thousands of unnecessary nephrectomies and change patient management decisions in treating clear cell renal cell carcinoma. Our peak sales estimate of US$120M may be conservative given Telix’s estimate of US$300-400M. The underlying target for this diagnostic (carbonic anyhdrase-9 or CAIX) potentially has an immediate, third adjacency in urology (urothelial cancer). We have covered potential as a marker of resistance to immune checkpoint inhibitors in previous research.
TLX101 and TLX66 – largest upside from modest investments. TLX101’s results in IPAX-1 support the progression of that asset into a registration trial in the second line setting (combination with external beam radiation therapy or EBRT). That interventional asset has potential corporate appeal for EBRT manufacturers. TLX66 (90Y-besolisomab) also delivered promising data. We like the leverage of that asset if developed and approved as a bone marrow conditioning agent. That utility is broadly applicable across a vast range of clinical situations where haematopoeitic stem cell transplant is indicated (haematologic malignancies, autoimmune indications, hereditary diseases).
Valuation. New price target of $10.35 per share equates to 20x FY23e revenue (WILSe: A$145M). Obvious caveats include major market approvals for ILLUCCIX which is the sole current source of revenue in our model. Our unrisked PT stands at $14.00 per share. Pipeline assets (TLX250, TLX66, TLX101) account for 11% of our risked SOTP valuation.
Silk Laser (SLA) - Price Target: $5.25 per share
Category leadership can re-rate 2x EV/EBITDA valuation points
Category leadership a goal for SILK in 2022. SLA trades on ~8.2x FY23e EBITDA which is in line with the sector median for small-midcap healthcare services. Category leaders (PSQ in dentistry; IDX in radiology) trade at a premium (~10x) as shown in Figure 5. In SLA we see a company bringing a corporatised approach to the non-surgical aesthetics (NSA) category which by and large has been a traditional franchising domain. The structure, systems and disciplines we associate with best-practice providers could exert competitive advantages in the NSA category which is more competitive and evolving quickly in terms of service mix. If SLA were to be valued at 10x FY23e its share price would be $5.25 per share.
Figure 5. Small-midcap heal
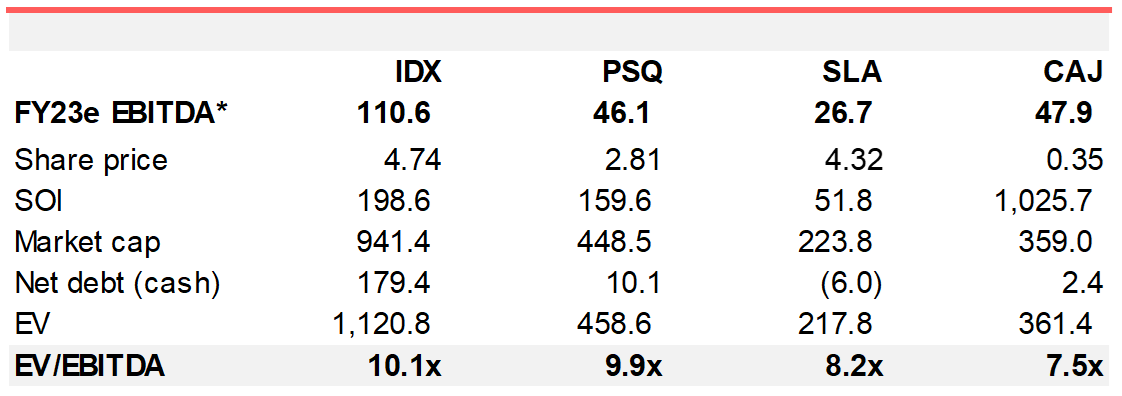
Source: Wilsons
Shrugging off caveats. Two cautionary factors to bear in mind as we move into FY22 are a) the likelihood of a difficult 1H comp and some reservations following a large acquisition this year. The dynamics playing into the 1H22 result (strong comparable period, headwinds on margin in delivering services booked through NSW/VIC lockdowns, modest exposure to start-up costs) are well flagged and captured in forecasts. Whilst some residual execution/integration risk remains following the ASC deal, industry feedback is positive on that transaction. Sector profitability is heavily skewed to the larger players (LCA and SLA/ASC). Market feedback suggests the clinic networks below these are not profitable enough to be attractive targets and more effectively competed away than acquired.
Zoom face, preventative Botox…all signal injectables growth. Looking to sales growth from AbbVie we see strong increases in both Botox™ and Juvéderm™ sales out of COVID (Figure 6), noting the 1Q20 acquisition of Allergan by AbbVie, as a contributor to this sales acceleration. Nevertheless, see this as indicative of the “preventative Botox” trend in Western markets. The use of Botox earlier in life as a preventative measure for wrinkles has seen the drug used more by the millennial generation with consumers in their mid-20’s and early 30’s a regular occurrence which would not have been the case a decade ago (Interesting NY Times article here). Social media has been a driver of trend adoption. Sales of both Botox™ and Juvéderm™ are ≥20% (CY19 vs CY20), with further acceleration into 2Q21 (+22-33% QoQ vs 1Q21). Silk is a clear beneficiary of this trend which we have seen with the injectables contribution to revenues expand YoY (≥40% FY21 vs 38% FY20) and injectables being a key growth driver for the business near term.
Figure 6. Abbvie’s Botox™ and dermal filler sales continue to see sustained double digit growth
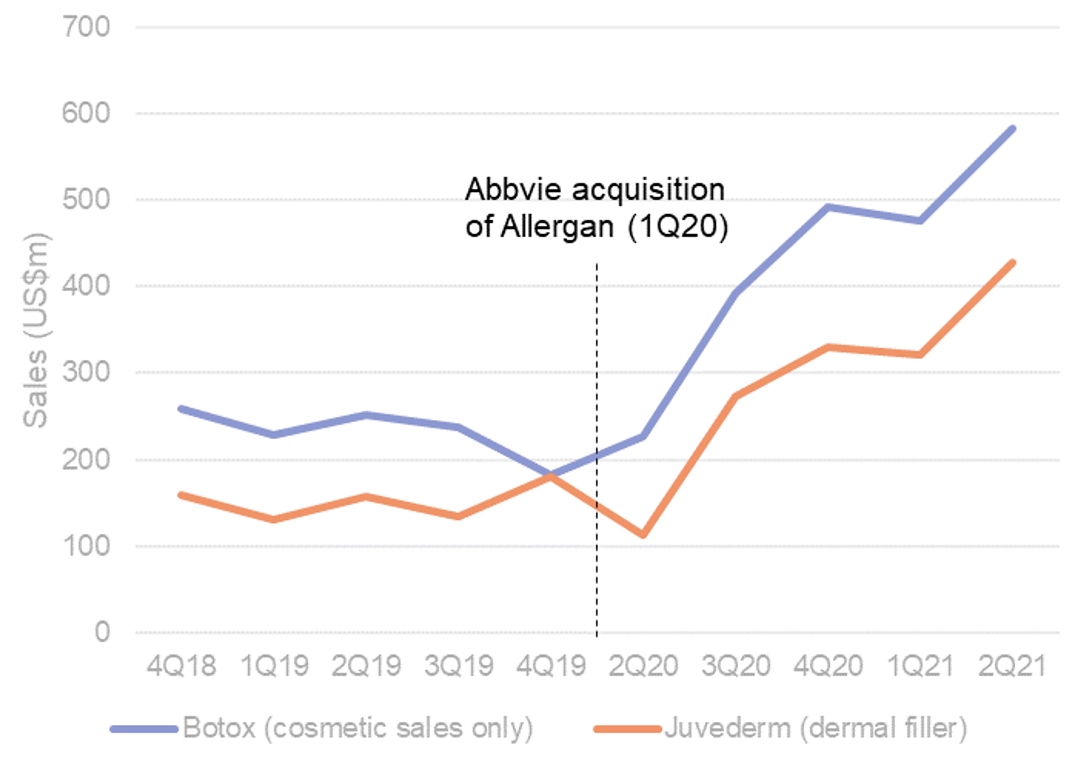
Source: AbbVie. Allergan.
Silk growing online; LCA makes online presence known. With an online presence being a key driver of sales for Silk and others during COVID lockdowns we interrogated website traffic data over this period, noting that 33% of Silk’s total laser/skin/body FY21 sales were generated online. In the past 7 months (April – Oct) we have seen silklaser.com.au gain viewership (clicks) (+13% average MoM growth) whilst recent acquisition australianskinclinics.com.au has held flat (+1% MoM). Net Silk + ASC online traffic average +2% MoM growth over this period (noting no data available for NZ which is 25% of ASC’s portfolio). During this same period competitor sites (laserclinics.com.au & clearskincareclincs.com.au) saw similar trends (+16% and +1% MoM respectively), noting that LCA was ~flat (+2%) excluding an Oct post-lockdown campaign. We would call out the volatility month to month in website traffic (Figure 7 overleaf), but also the sheer volume advantage LCA has with ~2x traffic vs Silk + ASC (490K v 250K visits/month). Interestingly, ~66% of SLA’s online traffic (as of Sept’21) feeds to an Afterpay portal speaking to client demographics. Geographic concentration of site visits aligns with states where each player has dominance (i.e. Silk in SA, QLD & WA; LCA in NSW & VIC). Silk has an opportunity to grow here in terms of visits but more importantly conversion, which we have already seen evidence of at the FY21 result.
Figure 7. Online website traffic levels for key Australian Non-surgical aesthetics players (Apr – Oct 2021)
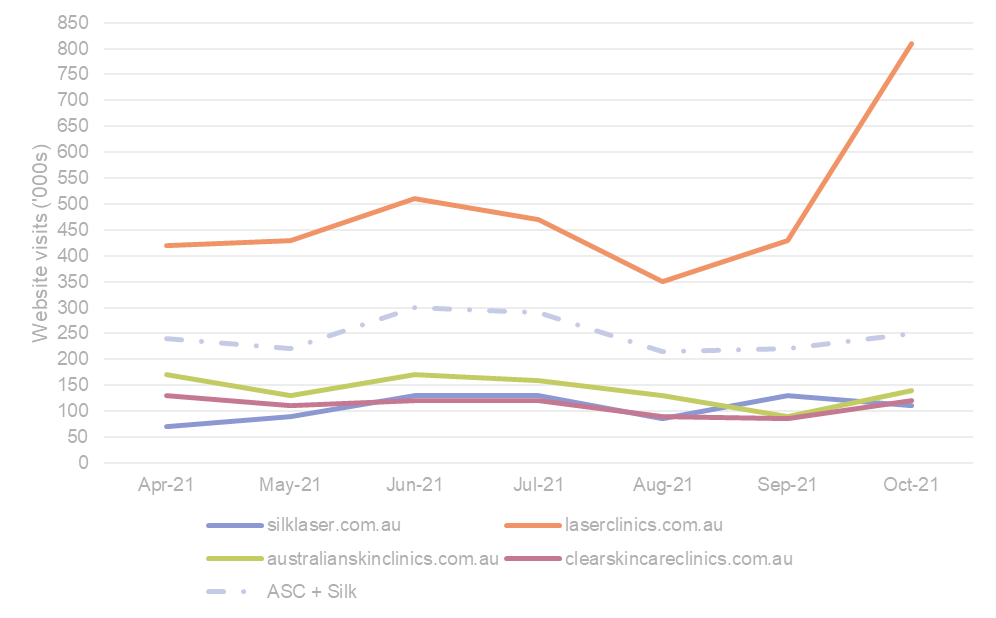
Source: SimilarWeb
Valuation. We’ve lifted our price target to $5.25 per share which is a 17% premium to DCF and more reflective of SLA assuming a level of category leadership over the next 12-18 months.
Realise your ambition
At Wilsons, we think differently and delve deeper to uncover a broad range of interesting investment opportunities for our clients. To learn more, please visit our website.
4 topics
6 stocks mentioned