Neuren – Where to from here?
At the time of our last Livewire publication (accessible here), Neuren (ASX: NEU) stood at a market cap of ~A$500m, with a share price of A$3.40. Trofinetide awaited FDA approval following a positive Phase 3 readout, Daybue (as trofinetide is now branded) had not yet launched in the US and Neuren’s pipeline candidate, NNZ-2591's Phase 2 trials had not begun. Our conclusion at the time was that, without assigning much value to NNZ-2591, Neuren could be conservatively worth around A$2.5b.
Fast forward two years and trofinetide has received FDA approval, and it has been successfully launched in the US under the brand name Daybue by Neuren’s partner, Acadia. Moreover, Acadia was awarded rest of world (RoW) rights to Daybue with Neuren receiving US$100m upfront, ~US$420m in milestones and mid-teens to low 20s royalties on future sales. Neuren has also recently released very promising data from its first Phase 2 trial for NNZ-2591 and finished FY23 with revenues of A$232m, net profit of A$157m and A$229m cash in the bank. The company’s share price has risen ~6x since our original wire and its market cap stands at ~$2.7b at the time of writing.
Another interesting development is that, while orphan drugs have been popular M&A targets for some time, neuroscience drugs are now back in vogue for big pharma M&A (see Bristol Meyers Squibb acquisition of Karuna, AbbVie’s acquisition of Cerevel and Biogen’s acquisition of Reata, discussed below). This positions Neuren well given their drugs straddle both therapeutic areas and is one of the reasons we spend time on Neuren’s acquisition value in this Wire.
The goal of this Wire is to assess the future of Neuren and NNZ-2591. As indicated, our previous report assigned limited value to NNZ-2591, however recent positive developments suggest that it is time to provide an update on our thesis. We believe that there is much more to come from this company following two years of substantial progress and there remains significant upside from current levels. While not the main focus, we also address some of the factual inaccuracies in a recent short report in the Appendix.
1. What is the upside left from Daybue?
A notable recent acquisition serves as a valuable benchmark for evaluating Neuren's potential, offering key insights into the valuation dynamics of companies specialising in rare disease drugs within the neurology space. Biogen, an American biotechnology company, recently acquired Reata Pharmaceuticals for US$7.3b (~A$11b). Table 1 below provides a detailed comparison between the primary approved drugs of the two companies (Skyclarys and Daybue), as well as NNZ-2591.
Table 1: Comparison of Skyclarys, Daybue and NNZ-2591 | |||
Company |
Reata |
Acadia |
Neuren |
Name |
Skyclarys |
Daybue |
NNZ-2591 |
Indication |
Friedreich's ataxia |
Rett Syndrome |
Phelan-McDermid Angelman Pitt Hopkins Prader-Willi
|
Age |
16 and older |
2 and older |
TBD |
Prevalence in the US |
~5,000 1 in 50,000 |
~6,000 – 9,000 1 in 20,000 |
> 100,000 total 1 in 8-15,000 1 in 12-24,000 1 in 11-41,000 1 in 10-30,000 |
Annual gross pricing estimate |
US$375,000 |
US$575,000 |
TBD |
FDA approval date |
Feb-23 |
Mar-23 |
|
Company pipeline |
Nil active trials |
3x Phase 2 trials |
4x Phase 2 trials |
Source: Biogen, Acadia, Neuren, KP Rx estimates (2024) |
There are a number of similarities between Skyclarys and Daybue that makes it a very good comparison. However, what are the differences and how does that affect value?
Mechanism of Action (MoA): Skyclarys treats a symptom (seizures) of Friedreich’s ataxia rather than the underlying disease whereas Daybue is approved to treat Rett Syndrome, the disease itself.
Label: The label for Skyclarys restricts its administration to patients aged 16 and above, imposing a notable limitation on its usage. In contrast, the label for Daybue permits treatment for patients aged 2 and older. Considering the average age of diagnosis for Rett Syndrome is approximately 2-3 years, this labelling provision does not significantly impede access to the medication.
Patient population: Acadia estimates the prevalent population to be approximately 6-9k patients which is considerably larger than the estimate of 5k patients for Friedreich's ataxia.
Pricing: We estimate the annual gross price for Daybue to be more than 50% higher than our estimate of Skyclarys’ gross price.
Tolerability: Skyclarys’ Phase 3 trial showed that the most common side effects were elevated liver enzymes (37% vs 2% in placebo), headache (37% vs 25%), nausea (33% vs 13%) and abdominal pain (29% vs 6%) (see here). Daybue’s common side effects were diarrhoea (82% vs 20%) and vomiting (29% vs 12%) (see here).
All key differences except tolerability outlined above points to Daybue being a more attractive drug than Skyclarys - the prevalent population is almost double, with Daybue eligible to treat a higher proportion of that population, while the drug is priced at a 50% premium. Skyclarys may be better tolerated than Daybue in the real world setting with diarrhoea on Daybue presenting a challenge to caregivers.
Additionally, as outlined in Chart 1 below, Daybue has outperformed Skyclarys following its commercial launch in the US, despite Skyclarys launching about 1 month earlier than Daybue.
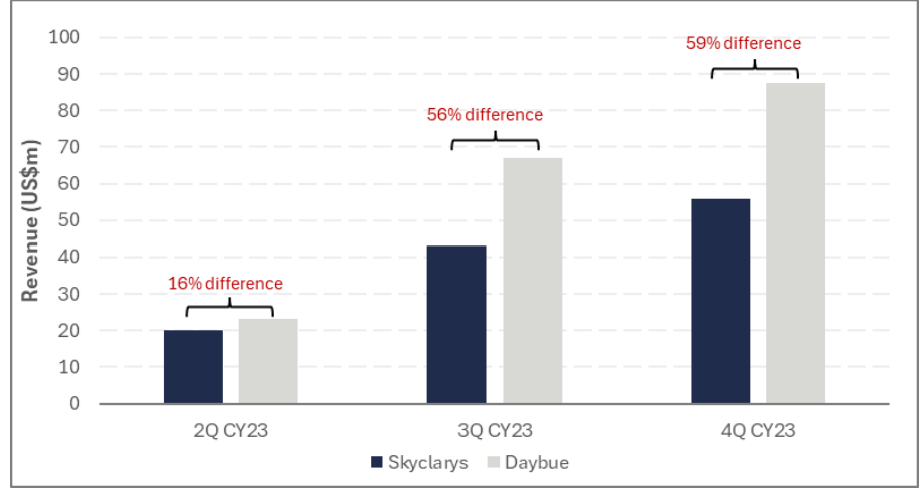
Data from the Phase 3 trial showed that Daybue is efficacious in 37.7% of patients (discussed in more detail in Appendix). Therefore, if we assume that 37.7% is the long term persistence rate and a net price of US$375k, Daybue's peak revenue would be approximately US$1.3b. Neuren would receive ~A$242m in royalties and ~A$540m (US$350m) in milestone payments.
The key unknown is the long term persistency rate of Daybue. Acadia have reported that persistency at month 7 is 63% which is considerably above what we had assumed given that only 37.7% of patients should see efficacy at the 3 month mark.
A key point to note is that Acadia holds global rights to Daybue for both Rett Syndrome and Fragile X. Consequently, an acquirer wanting to obtain Daybue would need to acquire Acadia rather than Neuren. Based on Acadia’s current market cap of ~US$3b and the insights derived from the above analysis, we view Acadia as representing a prime acquisition target. Such an acquisition would have significant positive implications for Neuren. Notably, Acadia currently lacks infrastructure outside of the US, which suggests that RoW rollout of Daybue may encounter delays compared to if Daybue rights were held by a large multinational pharmaceutical company.
2. How would an acquirer value NNZ-2591?
The Reata acquisition also provides a useful benchmark when thinking about what value big pharma might attribute to NNZ-2591, Neuren’s second compound currently progressing through clinical trials. While some of the comparisons for NNZ-2591 from Table 1 above are still to be determined, we can make the following observations:
Pricing: the most important determinant of pricing is efficacy (discussed in more detail below). We believe the efficacy of NNZ-2591 in relation to Daybue is a good way to think about pricing. That is, if NNZ-2591 delivers comparatively superior efficacy with a similar or better safety profile, it can be priced at a premium to Daybue.
Patient population: The key to NNZ-2591’s attractiveness to an acquirer is its potential use in many orphan and non-orphan diseases. The current Phase 2 trials are exploring four different orphan diseases which have a total prevalent population of greater than 100,000 patients in the US (as shown in Table 1).
Future potential: Within orphan neurological diseases, there are many other indications that Neuren or an acquirer could pursue. Outside of orphan diseases, we see potential for this drug in neurodegenerative diseases such as Alzheimer’s disease and other dementias. In addition, we see a solid scientific rationale for this drug being trialled in autism. Given this broad applicability of the mechanism of action, NNZ-2591 is a pipeline in a drug.
It's worth noting one significant complication in the potential acquisition of Neuren for NNZ-2591; Acadia holds the global licenses for NNZ-2591 in Rett Syndrome and Fragile X. Consequently, any acquiring entity would need to collaborate closely with Acadia through a steering committee for NNZ-2591's development. This partnership could pose a considerable obstacle to potential acquirers, greatly complicating both the developmental and commercial aspects of NNZ-2591. For instance, detailed plans for which indication will be sought for approval first, the market launch strategy, marketing approach etc. would all require close coordination with Acadia. Furthermore, unforeseen issues such as adverse reactions in a trial of a specific patient population or unfavourable pricing in certain markets could significantly impact not only the drug's development trajectory, but also the approved label, price and eventual commercial success.
These concerns would be mitigated if the acquirer were to acquire both Neuren and Acadia. This would grant the acquirer total global rights to both Daybue and NNZ-2591 for all indications without any obligation to pay royalties.
3. What is the potential for NNZ-2591?
Neuren recently announced topline data from its Phase 2 clinical trial in patients with Phelan McDermid Syndrome (PMS) which is the first set of data we have seen of NNZ-2591 in patients.
The headline results were very impressive with positive data across a range of efficacy endpoints. Importantly, the gastrointestinal (GI) side effects that are seen with Daybue are not present in this trial. This presents a significant advantage for the practical application of this drug in a real-world setting.
The Phase 2 trial of NNZ-2591 for the treatment of PMS was an open label trial, meaning there was no placebo comparator, and the trial was not blinded. When assessing a trial like this we need to:
Assess what the placebo response might be in this patient population. This is particularly important for a condition like PMS as the efficacy measures are based on subjective parent or physician assessed rating scales. To do this we generally look for other placebo-controlled blinded trials and see what the response rates are in the placebo arm.
Compare these results with findings from other completed Phase 2 studies to assess how this result compares and to better understand the probability of success. Specifically, we aim to assess whether the outcomes observed in Phase 2 trials of other drugs were corroborated in Phase 3 trials, and if not, to ascertain the underlying reasons for any disparities.
There are some very important caveats that we will call out upfront in our analysis of this efficacy data.
The trial’s sample size is very small, 18 patients. PMS is a heterogeneous disease meaning symptoms are varied, progression is sporadic and different for all patients.
As stated above, the trial is not blinded and does not have a comparator arm. When assessing such a trial we must make cross-trial comparisons which are normally very difficult to make. In PMS, very few clinical trials have been completed. In fact, Clinicaltrials.gov has only 14 trials listed for PMS of which only 8 are interventional trials assessing a treatment for PMS all with very small sample sizes. These factors further complicate a cross trial comparison.
No Phase 3 trials have been conducted in PMS. Thus, we are unable to compare how a drug’s Phase 2 results and Phase 3 results compare.
As a reminder, NNZ-2591’s primary mechanism of action is to increase the levels of insulin like growth factor 1 (IGF-1) in the brain by ‘freeing’ IGF-1 from its binding protein, thereby allowing IGF-1 to bind to its receptor. With that in mind, the most relevant trials we found for comparison and assessment of the NNZ-2591 Phase 2 trial were published in Molecular Autism (Kolevzon et al, 2002). These two randomised, blinded, placebo-controlled trials evaluate the therapeutic effect of IGF-1 in PMS patients measured at 12 weeks.
Interestingly, these trials showed that IGF-1 might have a therapeutic benefit in PMS patients. As a reminder, Daybue has a similar mechanism of action to NNZ-2591. Thus, there is now a growing body of evidence that increasing the levels of IGF-1 may have a therapeutic benefit across many diseases of the brain. This reflects positively on NNZ-2591 given that it is designed to increase the levels of IGF-1 in the brain.
Table 2: Topline results from NNZ-2591 and IGF-1 trials | |||
|
NNZ-2591 Phase 2 |
Trial 1 |
Trial 2 |
Sample size |
18 |
9 |
10 |
Age |
3-12 (median 8.3) |
5-15 |
5-15 (median 8.7) |
Design |
Open label, single arm |
Randomised, blinded, placebo-controlled crossover |
Randomised, blinded, placebo-controlled crossover |
Intervention |
NNZ-2591 |
IGF-1 |
IGF-1 |
CGI-I |
2.4 |
NR |
Active arm: 2.6 Placebo arm: 3.1 |
CGI-S |
NR |
NR |
Active arm: -0.2 Placebo arm: -0.1 |
ABC-Hyperactivity |
-6.3 |
NR |
Active arm: -6.2 Placebo arm: -2.8 |
ABC-Irritability |
-4.9 |
NR |
Active arm: -3.8 Placebo arm: -5.5 |
ABC-Social withdrawal |
-4.8 |
Active arm: -8.2 Placebo arm: -1.5 |
Active arm: -5.5 Placebo arm: -6.3 |
Source: Neuren, Kolevzon et al (2002) |
These trials allow us to observe the placebo response in a blinded randomised controlled trial to get an idea of what the placebo response might be in the NNZ-2591 trial. This data indicates that it is unlikely a CGI-I of 2.4 achieved in the NNZ-2591 trial is due wholly to a placebo response.
The Chart below shows that the CGI-I response with NNZ-2591 is positively skewed with 10/18 patients demonstrating a ‘Much Improved’ or ‘Very Much Improved’ score. This further adds weight to the argument that NNZ-2591 is having a therapeutic response in PMS.
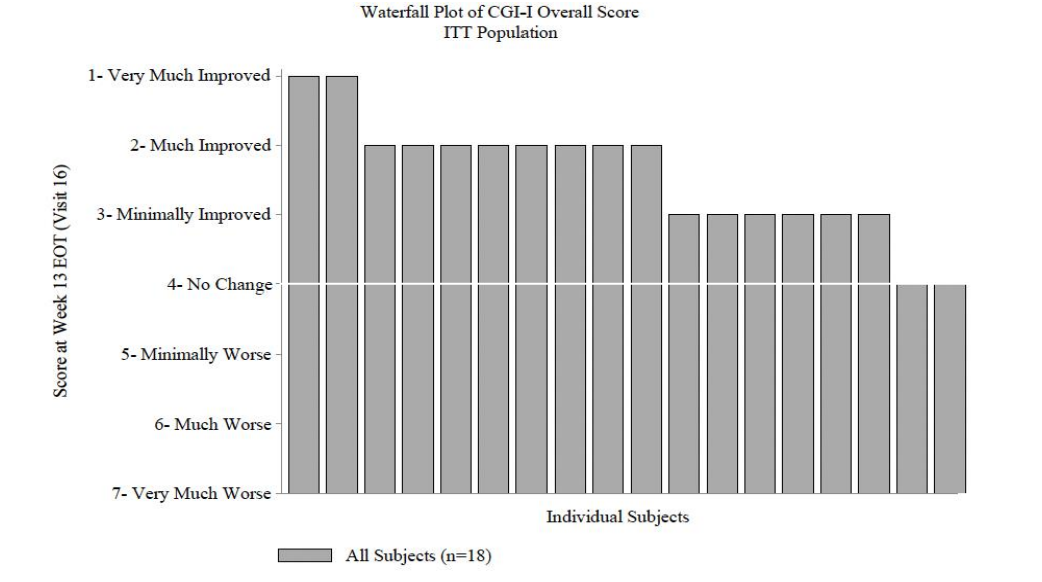
While this data is positive and indicates that NNZ-2591 is having a therapeutic effect, the data is derived from small sample sizes. We eagerly await results from Phase 2 trials in Angelman’s, Prader Willi and Pitt Hopkins Syndromes in the coming months.
4. Putting it together
Table 3 below looks at how our views on valuation have changed since we published our last Wire and incorporates the new information we have discussed above in relation to Daybue.
Table 3: Daybue valuation for Neuren | ||
Value components of Daybue |
Changes to our view |
Value |
Neuren’s 1/3 share of the priority review voucher (PRV) |
Acadia recently announced that pimavanserin has failed in a Phase 3. This was the last late-stage trial Acadia was running making it more likely that Acadia will now sell the PRV. No change to the value. |
A$46m based on recent transactions |
Commercial milestones based on sales in North America |
No change |
A$493m possible in total |
Base Case Royalty Stream: 11.3% blended royalty on US$560m of Acadia sales x 70% margin after tax x 10x multiple (considering patent life and paediatric orphan drug exclusivity) Upside Case: 3k patients which is ~30% of total patient population and at US$375k price |
|
A$682m – A$1,575m |
Value of US market |
|
A$1.2b - A$2.1b |
Sales milestones in Europe |
|
A$315m |
European market (3,000 patients, 50% price discount relative to the US, 20% royalty, 70% net margin x 10x multiple) |
|
A$1.2b |
Total Value |
|
A$2.7b - A$3.6b |
While the gap between Neuren’s market cap and intrinsic value has closed since our last Wire, we still see Neuren as undervalued based on the Daybue opportunity in Rett Syndrome alone.
In Table 4 below we look at the revenue potential for NNZ-2591 in the US in the four indications currently being studied. The two key assumptions made are 30% peak penetration and US$375k pricing, both in line with Daybue. We note that both assumptions are conservative given that NNZ-2591 may have broader usage given the better tolerability profile and price could be at a premium to Daybue if the results show a more meaningful improvement in symptoms. We assigned a higher probability of success (POS) to Phelan-McDermid as the Phase 2 is completed with strong results and lower POS for Prader-Willi due to the competition from other products in development and difficulty treating the disease.
Table 4: NNZ-2591 valuation | |||
Indication |
Market assessment |
Peak revenue |
Risk-adjusted peak revenue |
Phelan McDermid |
Patient population: ~40k. Assume 30% peak penetration. Price: US$375k
|
A$6.9b |
A$2.1b 30% POS |
Angelman |
Patient population: ~27k. Assume 30% peak penetration. Price: US$375k
|
A$4.6b |
A$692m 15% POS |
Pitt Hopkins |
Patient population: ~29k. Assume 30% peak penetration. Price: US$375k
|
A$5b |
A$755m 15% POS |
Prader Willi |
Patient population: ~32k. Assume 30% peak penetration. Price: US$375k
|
A$5.5b |
A$554m 10% POS |
Total |
|
A$22b |
A$4.1b |
Generally, an acquirer would be a company that already has a presence in the given field with an established sales force and back office to ‘plug’ NNZ-2591 into. As such the marginal cost to commercialise NNZ-2591 to an acquirer would not be significant. Simplistically, in this scenario an acquisition might be a multiple of peak revenue with a discount to reflect the risks and costs of clinical development and time value of money. The typical price to sales ratio for pharma is approximately ~3x. When applied to our risk adjusted peak revenue estimate, this results in a risked valuation for NNZ-2591 at ~US$12.3b.
At the time of acquisition, Skyclarys' projected peak revenue stood at ~US$1.5b by 2030, yielding a peak sales/price multiple of around 4.8x. Employing this multiple as a benchmark for assessing our valuation, if we apply it to our forecasted peak revenue for NNZ-2591 of US$22b (as per table 4) and factor in a significantly conservative discount of 90-95% to account for clinical and regulatory risks, the resultant acquisition price still ranges between ~US$5.3b to US$10.6b.
The discussion above ignores the value of NNZ-2591 in Rett Syndrome and Fragile X which Acadia has licensed. There are no costs associated with these programs for Neuren and act as a free option if the programs are developed and launched.
5. Key risks
#1: As we discussed above, long term persistency of patients on Daybue is the key risk to Neuren’s valuation in the short term. Although current data suggests that adherence rates exceed those observed in clinical trials and above our expectations, it's important to recognise that the date is still early. We will keep a close eye on Acadia's quarterly updates for any new insights.
#2: The RoW rollout of trofinetide presents potential execution challenges given Acadia has no infrastructure outside of the US. However, we see this risk as mitigated given the lack of approved treatments in Rett Syndrome, coupled with a significant unmet medical need and the drug’s successful launch in the US. We continue to assess how Acadia is building up its RoW business with a specific focus on Europe.
#3: Clinical development risk of NNZ-2591. Notwithstanding the strong preclinical and Phase 2 results, significant clinical risk exists for any drug at Phase 2 stage in neurology. That said, we believe that Neuren is undervalued even when ignoring the NNZ-2591 opportunity, thus we view NNZ-2591 as a ‘free option’ at the current market valuation.
#4: Competing treatments in development can pose a risk to Daybue. Taysha Therapeutics is currently trialling a gene therapy for Rett Syndrome which has entered Phase 1. Early data from 2 patients shows that the drug is safe, but it is too early to assess efficacy. Similar for NNZ-2591, numerous therapies are in development for the disease states.
6. Summary
Since our last update, there’s been a notable shift in the landscape of pharmaceutical M&A, which is one of the reasons we have spent time on Neuren’s acquisition value in this Wire. While orphan drugs have consistently garnered interest as targets for M&A, there has been a significant resurgence in the appeal of neuroscience drugs among major pharmaceutical companies. This trend is exemplified by several high-profile acquisitions, including Bristol Meyers Squibb's acquisition of Karuna, AbbVie's purchase of Cerevel, and Biogen's takeover of Reata. This evolving market dynamic positions Neuren advantageously, as its portfolio spans both the orphan drug and neuroscience therapeutic domains, thus accentuating its potential as a strategic target for future M&A endeavours.
Over the last two years, Neuren has achieved important milestones, with the company providing a much need treatment for Rett Syndrome and delivering significant value to its shareholders. We continue to see a bright future for Neuren driven by the clinical development of NNZ-2591, Neuren’s jewel in the crown. The early trial data for NNZ-2591 has prompted a reassessment of our thesis, leading us to earnestly contemplate the value potential it holds. We are eager to review the results from the upcoming Phase 2 read outs, as we firmly believe in the tangible pathway for substantial value realisation for patients, Neuren and its shareholders in the foreseeable future.
References
- May D et al (2023) Epidemiology and patient journey of Rett syndrome in the United States: a real-world evidence study. BMC Neurol. 2023 Apr 4;23(1):141. doi: 10.1186/s12883-023-03181
- Kolevzon A et al (2002) Clinical trial of insulin-like growth factor-1 in Phelan-McDermid syndrome. Mol Autism. 2022 Apr 8;13(1):17. doi: 10.1186/s13229-022-00493-7
- Neul JL et al (2024) Trofinetide for the treatment of Rett syndrome: a randomized phase 3 study. Nat Med. 2023 Jun;29(6):1468-1475. doi: 10.1038/s41591-023-02398-1
Appendix: Short Report
Some readers may be aware that a short report was released earlier this year. While in general we would prefer not to dwell on such reports, there are a number of factually incorrect and highly sensationalist claims in the report which are worth touching on. We’ve addressed a number of these below.
Poor response and side effects: The report emphasises, based on chart 3 below, that only 13% of patients showed a “much improved” CGI-I score (which assesses how much the patient's illness has improved or worsened relative to a baseline state at the beginning of the intervention), while 92% experience adverse events such as diarrhoea, vomiting, and seizures. This information was already known from Acadia’s Phase 3 results. From conversations we have had with key opinion leaders (KOLs), a one-point change in the CGI-I is considered clinically meaningful. Thus, the data from the Phase 3 trial showed that 37.7% of patients had a clinically meaningful response to Daybue over 12 weeks.
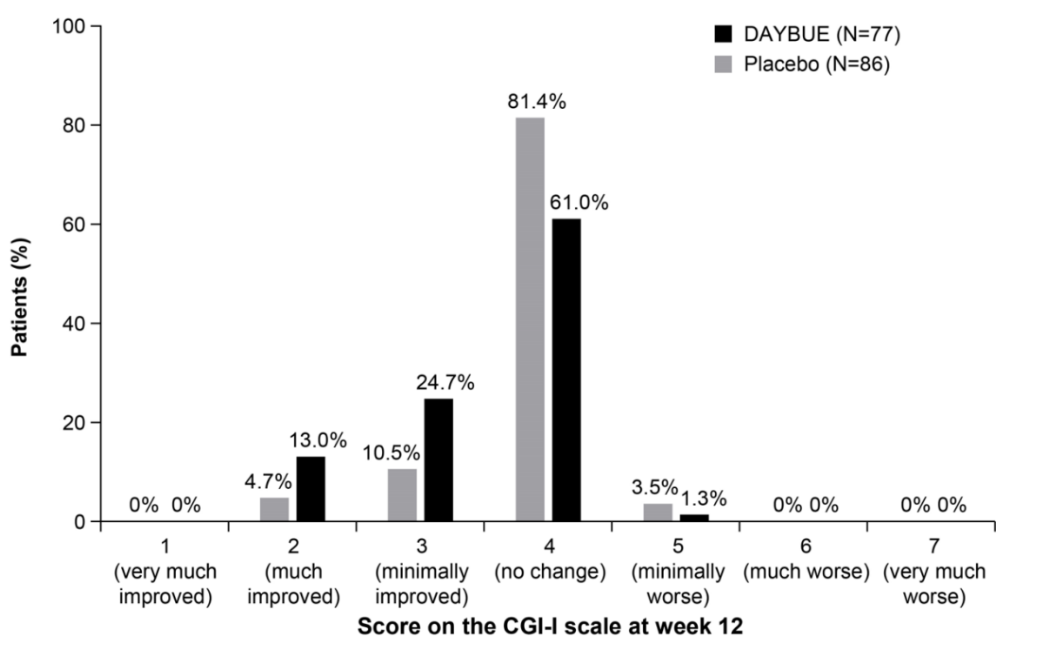
Discontinuation rates are higher than reported: The report draws the conclusion, following discussions with a number of physicians, as well as Facebook posts from parents, that discontinuation rates are misrepresented by Acadia. For reference, from its latest earnings call, Acadia showed a persistency rate at 7 months of 63%, and that it continues to see data improvements over all time periods. While it will require another few quarters to form a clearer view on persistency rates, our own expectations of retention rates were previously much lower than those being reported by Acadia. As per Chart 3 above, given that only 37.7% of patients should see efficacy at the 3-month mark, a 63% persistency rate at month 7 is well above what we had expected at this stage.
It is important to note that the clinical trial setting is much harsher than the real world. Daybue is being utilised in the real world with the aim of reducing the burden of side effects which was not possible during the clinical trial:
Patients titrate their dose up slowly to a target dose. The titration can be paused if side effects present and that dose level is held until the side effects resolve. Patients on the clinical trial were required to start on the highest dose with no dose titration allowed.
The Phase 3 trial did not have a diarrhoea management plan at the commencement of the trial which meant that parents had no guidance on how to mitigate this side effect. Rett Syndrome is a disease characterised by constipation and, as such, most patients are on laxatives. In the real world setting we are hearing various strategies that work for individual patients including removing laxatives, changing diets, supplementing certain foods with the administration of Daybue. In addition, parents can manage this side effect at a lower dose as they titrate the dose up.
Daybue’s safety profile mischaracterised: The report claims that roughly 1 of every 10 to 11 Daybue patients are hospitalised as a result of the drug. Such statements are sensationalist and factually incorrect. Acadia are obligated to disclose to the FDA all reports they receive about patients that are on or have taken Daybue, regardless of whether the incident was caused by Daybue or not. All this data is collected in the FDA Adverse Event Reporting System (FAERS) Database and made publicly available. We encourage readers to look through this data for themselves (see here). The hospitalised patients are reported to have had conditions such as pneumonia, urinary tract infections, influenza and many other conditions that are unlikely to be caused by Daybue. In the clinical trial only two side effects were significantly higher in the Daybue arm vs placebo, which is diarrhoea and vomiting.
Rett Syndrome patients have a range of co-morbidities and complications arising from their condition that can require hospitalisation. We encourage readers to review the data from a real-world patient journey study which categorises this in detail (May et al, 2023).
Interestingly, diarrhoea occurred in approximately 20% of patients in the placebo arm of the Lavender Phase 3 trial in a condition where constipation is expected to occur in over 80% of patients. This might indicate that the drug formulation has inherent properties that causes diarrhoea independent of the action of Daybue. For instance, the fluid may have a high osmolality which draws water into the bowels potentially causing diarrhoea. We would be very surprised if Acadia were not working on a new formulation of Daybue that would do away with this liquid formulation thus reducing the burden of diarrhoea. This would be a smart lifecycle management strategy which may provide additional patent protection on the new formulation protecting the Daybue franchise for longer. This might also increase the number of patients who can tolerate Daybue thus increasing the peak revenue forecasts depending on its impact on the rates of diarrhoea.
New Daybue scripts peaked in August 2023: The report states that “our analysis of FAERS data also suggests that new patient starts peaked this summer.” It should be noted that the FAERS data cannot be used to estimate the number of patients on a drug and any attempt to do so is careless. A parent is very unlikely to call Acadia or the FDA every month to report the same side effect (e.g., diarrhoea) and, as clinical management improves, the need to seek assistance from sources rather than your clinician will decrease. Generally, with any new drug launch, it is common to see a spike of adverse event reporting around the launch and then a decrease. Regardless, the claim that Daybue sales has peaked in August is false as patient numbers continued to climb to the end of 2023.
4 topics
3 stocks mentioned

